File
advertisement

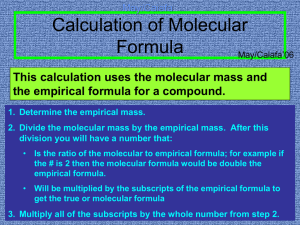
3day8 – Molecular Formula The molar mass of a molecular compound is always a whole number multiple, x, of the molar mass of the empirical formula. You can determine the molecular formula by 1. Find x by dividing the molecular mass by the empirical mass 2. Multiply the subscripts in the empirical formula by this number to get the molecular formula Example: A white powder is found to have an empirical formula of P2O5. The compound has a molar mass of 283.88g. What is the compound’s molecular formula? 1. Find molar mass of the empirical formula P= 2(30.97) = 61.94 O = 5(16.00) = 80.00 MM = 141.94 2. Divide MM by empirical MM 283.88/141.94 = 2 3. Multiply subscripts P2O5 P4O10 Example: A compound has an experimental molar mass of 78.12 g/mol. Its empirical formula is CH. What is its molecular formula? Molar mass of the empirical formula C = 1(12.01) = 12.01 H = 1(1.01) = 1.01 MM = 13.02 Find multiple: x = 78.12/13.02 = 6 Multiply subscripts: CH C6H6 Class work: Do the worksheet Homework: p. 300 practice #2. P. 300 #2, 4, 6-8