Mole Concept Review: Chemistry Worksheet
advertisement

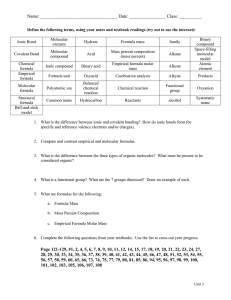
Unit 3 Review: The Mole 1. 2. 3. 4. I can name ionic and molecular compounds using roman numerals or prefixes when necessary. I can calculate molar mass and percent composition. I can convert between grams, moles, and particles of a substance. I can calculate empirical and molecular formulas. I Can #1: Chemical Formulas For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. 1) Na2CO3 _________________________________________ 2) P2O5 _________________________________________ 3) NH3 _________________________________________ 4) FeSO4 _________________________________________ 5) SiO2 _________________________________________ 6) GaCl3 _________________________________________ 7) CoBr2 _________________________________________ 8) B2H4 _________________________________________ 9) CO _________________________________________ 10) P4 _________________________________________ For each of the following questions, determine whether the compound is ionic or covalent and write the appropriate formula for it. 11) dinitrogen trioxide _________________________________________ 12) dinitrogen pentaoxide_________________________________________ 13) carbon tetrahydride _________________________________________ 14) lithium acetate _________________________________________ 15) phosphorus trifluoride _________________________________________ 16) vanadium (V) oxide _________________________________________ 17) aluminum hydroxide _________________________________________ 18) zinc sulfide _________________________________________ 19) silicon tetrafluoride _________________________________________ 20) silver phosphate _________________________________________ I Can #2: Molar Mass and Percent Composition 1. What is Avogadro’s number? 2. Determine the molar mass of Al. 3. Determine the molar mass of C12H22O11 4. Determine the percent composition of the Cu(OH)2 to the nearest tenth percent. 5. What is the percent by mass of hydrogen in aspirin, C9H8O4? If you have 500. mg of aspirin, what is the mass of hydrogen? I Can #3: Conversions 1. How many atoms are present in 5.06 moles of the element silicon? 2. How many molecules are in 8.04 moles of NaCl? 3. How many ions are in 7.75 moles of Au2+? 4. How many moles are in a container of 98.6 grams of KCl? 5. How many molecules are there in 6.07 g of Ag2CO3? 6. What is the mass of 1 atom of lead? 7. What is the mass in grams of 5.74x1024 molecules of AlCl3 I Can #4: Empirical and Molecular Formulas 1. Determine the empirical formula for a compound that contains 1.56 g of copper and 3.92 g of bromine. 2. Which of the following compounds, if any, are written in empirical form: H2O2, C6H12O6, CCl4? 3. What is the empirical formula of a compound which consists of 89.14% gold and 10.80% oxygen? 4. The empirical formula of a substance is CH2O. The molar mass is 180 g/mol. What is the molecular formula? 5. A 3.585 g contains 1.388 g of C, 0.345 g of H, and 1.850 g O and its molar mass is 62 g. What is the molecular formula of the substance?