CH107 FALL SEMESTER 2011
advertisement

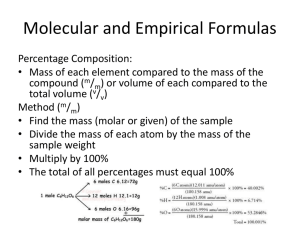
CH107 SPECIMEN EXAM-TYPE QUESTIONS (4) Chapters 5/6 The questions are worth equal points Show working for questions 4 and 5 1. (a) Barium is a reactive metal. Write the combination reactions that occur when barium metal reacts with oxygen, nitrogen, and fluorine, and balance the equations. (b) Combustion reactions are important throughout science. Write a balanced equation for the combustion of sucrose (C12H22O11) in excess oxygen. 2. Acid rain is responsible for damage to many plants and man-made structures (especially if composed of marble or limestone). The major components of acid rain are sulfuric and nitric acids, which are formed by the atmospheric oxidation of combustion products SO 2 and NO2, respectively. Write a balanced overall equation for each of these two oxidations. 3. Decide which of the following would lead to spontaneous reactions and write net ionic equations, where appropriate. (a) A chromium wire is placed in a solution of nickel(II) sulfate. (b) A piece of silver is placed in a copper(II) sulfate solution. (c) A nickel strip is immersed in a silver nitrate solution. 4. The empirical formula for sodium dithionate is NaO 3S. Based on the empirical formula, address the following questions. (a) If the molar mass of the compound is 206.11 g/mol, what is the molecular formula of sodium dithionate? (b) What is the percent composition of Na in the compound? (c) What is the mass ratio between O and S (O:S) in the compound? (d) What is the mass, in grams, of 2.30 x 1022 formula units of this compound? (e) How many atoms of S are in a 2.35 g sample of the compound? 5. Ascorbic acid (vitamin C) is composed of (by mass) 36.7% C, 4.6% H and 54.6% O. Its molar mass is 176.1 g/mol. Determine its empirical and molecular formulas. 1 Solutions 1. 2Ba(s) + O2(g) 3Ba(s) + N2(g) Ba(s) + F2(g) 2BaO(s) Ba3N2(s) BaF2(s) 2. 2SO2(g) + O2(g) + 2H2O(l) 2H2SO4(aq) 4NO2(g) + O2(g) + 2H2O(l) 4HNO3(aq) (these reactions actually occur by different mechanisms, all of them stepwise) 3. (a) 2Cr(s) + 3Ni2+(aq) 2Cr3+(aq) + 3Ni(s) (b) No reaction (Ag lies below Cu in the activity series) (c) Ni(s) + 2Ag+(aq) Ni2+(aq) + 2Ag(s) 4. (a) Na2O6S2 (b) (46.0/204.1) x100 = 22.5% Na (c) O:S = 1.5:1 (the atom ratio is 3:1, but the atomic mass of S is 2 x atomic mass of O) (d) (2.3 x 1022 units /6.02 x 1023/mole) x 206.11 g/mole = 7.87 g Na2O6S2 (e) 2.35 g x (mole/206.11 g) x (6.02 x 1023/mole)(2 atoms S/1 formula unit) = 1.37x1022 atoms S 5. Empirical formula: C3H4O3 Molecular formula: C6H8O6 (From the elemental composition data, the empirical formula is C3H4O3, whose formula mass is only 88.1 g/unit; one half the molar mass. Hence the molecular formula is double the empirical formula) 2