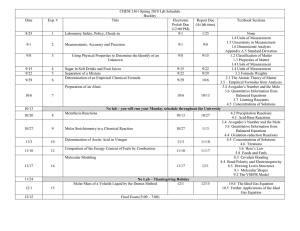
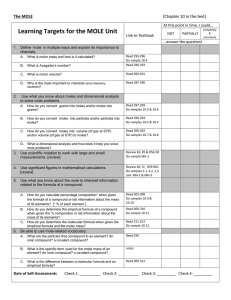
Chapter 10 Reading Questions
advertisement

Chapter 10 Reading Questions Section 10.1 1. 2. 3. 4. 5. 6. 7. 8. 9. What three ways can you measure matter? Do Practice problem 1 (p.289) using conversion factors. What is a mole? What is Avogadro’s number? What are the three types of representative particles? What is an atomic mass unit? What is the mass of a mole of an element? What is another definition of a mole? How do you calculate molar mass of a compound? Section 10.2 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. What can you use the molar mass of a compound for? What relationship do you use for a conversion factor to turn grams into moles? Do practice problem 18. How do volumes of a mole of liquid and gas compare? How are the volumes of gases different? What is Avogadro’s hypothesis? What is standard temperature and pressure? What do you use for a conversion factor for volume and moles? Do practice problem 20. What equation will we use to relate molar mass and density/ Section 10.3 1. 2. 3. 4. 5. 6. 7. 8. 9. What is percent composition? What do you use to calculate the mass of each element of a compound? What is the sum of the masses? How do you calculate the percent mass of each element in a compound? What do you use the percent composition of a compound for? How do you do this? What does the empirical formula give you? How is the empirical formula related to the molecular formula? How are they different? What is the empirical formula mass and how do you use it?