Chapter 4 Compounds and Their Bonds
advertisement

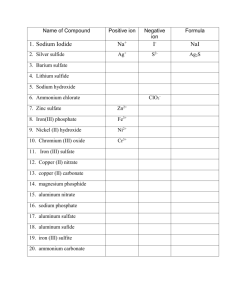
Polyatomic Ions an electrically charged group of two or more covalently bonded atoms that functions as a single ion NH4+ ammonium OH- hydroxide NO3- nitrate NO2- nitrite CO32- carbonate ______________ HCO3- hydrogen carbonate (bicarbonate) More Polyatomic Ions Sulfur SO42- sulfate SO32- sulfite HSO4- hydrogen sulfate HSO3- hydrogen sulfite Phosphate phosphite PO33- ____________ PO43- phosphate HPO42- hydrogen phosphate _______________________________ H2PO4- dihydrogen phosphate Naming Ternary Compounds Contain at least 3 elements Name the nonmetals as a polyatomic ion Examples: NaNO3 Sodium nitrate K2SO4 Potassium sulfate Al(HCO3)3 Aluminum bicarbonate or aluminum hydrogen carbonate Learning Check Match each set with the correct name: A. Na2CO3 1) magnesium sulfite MgSO3 2) magnesium sulfate MgSO4 3) sodium carbonate B. Ca(HCO3)2 CaCO3 1) calcium carbonate 2) calcium phosphate Ca3(PO4)2 3) calcium bicarbonate Solution A. B. Na2CO3 MgSO3 3) sodium carbonate 1) magnesium sulfite MgSO4 2) magnesium sulfate Ca(HCO3)2 3) calcium bicarbonate CaCO3 1) calcium carbonate Ca3(PO4)2 2) calcium phosphate Naming Ternary Ionic Compounds NaNO3 CaSO4 CuSO3 (NH4)2O LiCN Fe(OH)3 (NH4)2CO3 NiPO4 Ionic Names Consider the following: Does it contain a polyatomic ion? -ide, 2 elements no -ate, -ite, 3+ elements yes Does it contain a Roman numeral? Check the table for metals not in Groups 1 or 2. No prefixes! Ionic Names potassium chloride K+ Cl- KCl Mg(NO3)2 CuCl2 magnesium nitrate Mg2+ NO3copper(II) chloride Cu2+ Cl- Ionic Names NaBr sodium bromide Na2CO3 sodium carbonate FeCl3 iron(III) chloride Writing Formulas Write the positive ion, then write the negative ion The charges have to add up to zero Write the number of ions needed as subscripts Put more than one polyatomic ions in parenthesis If anion ends in -ate or -ite it is polyatomic Don’t show charges in the final formula. Learning Check A. aluminum nitrate Al(NO3)3 B. copper(II) nitrate Cu(NO3)2 C. Iron (III) hydroxide Fe(OH)3 D. Tin(IV) hydroxide Sn(OH)4 Write the formulas for Lithium sulfide Tin(II) oxide Tin(IV) oxide Magnesium fluoride Copper(II) sulfate Iron(III) phosphide Gallium nitrate Iron(III) sulfide Barium nitrate Ammonium chloride ammonium sulfide