Molecular formulas - Madison County Schools
advertisement

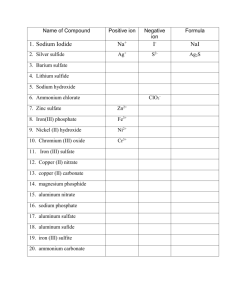
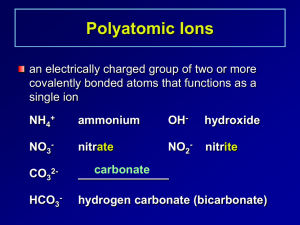
Writing Formulas Review Ionic Compounds 1) a metal and nonmetal compound. Metals are on the left of the periodic table, nonmetals are on the right of the stair step line. a) The metal forms a positive ion called a cation. b) The nonmetal forms a negative ion called an anion. c) The cation is always written first in a compound. 2) All group 1 metals form a 1+ ion, all group 2 metals form a 2+ ion, B and Al form 3+ ions, Group 15 down to the metalloid line form 3- ions, Group 16 down to the metalloid line form 2- ions, and Group 17 form 1ions. All other elements may form more than one ion except Ag, Zn, and Cd, which always form Ag1+, Zn2+ and Cd2+ 3) Polyatomic Ions – YOU MUST MEMORIZE!!!! Ammonium, nitrite, nitrate, sulfite, sulfate, hydrogen sulfate (bisulfate), hydroxide, cyanide, phosphate, hydrogen phosphate (biphosphate), dihydrogen phosphate, carbonate, hydrogen carbonate (bicarbonate), hypochlorite, chlorite, chlorate, perchlorate, acetate, permanganate, dichromate, chromate, peroxide, oxalate (C2O42-), phosphite (PO33-) a) Note: You must memorize the formula, the charge and the name! 4) Transition metals and metal below the metalloid line on the periodic table can have more than one charge. You will know the charge by the Roman numeral in the formula’s name. Ex. Iron (II) oxide = Fe2+, Lead (IV) oxide = Pb4+. Once you know the cation and anion’s charges, you may put the compound’s formula together by balancing the charges. The charges must add up to zero in the final compound. The compound may not have any charges on it. You may use the criss-cross method if you like. However, you must remember to reduce the subscripts. Ex. Fe3+ and O2- Fe2O3 2 and 3 cannot be reduced, the formula is correct. Ex. Pb4+ and O2- Pb2O4; However 2 and 4 can be reduced, so the correct formula is PbO2. Remember, if you need more than one polyatomic ion you must put parenthesis around it and put how many you need outside of the parenthesis. You may never change any subscript in the polyatomic ion. Molecular formulas Made up of two nonmetals You will recognize their names because they use prefixes. Mono-1, di-2, tri-3, tetra-4, penta-5, hexa-6, hepta-7, octa-8, nona-9, deca-10. Whatever prefix the element has is how many atoms are in the formula. Diphosphorous pentoxide = P2O5. Acids Has the word acid attached to it. Will always have an H+ as the cation. If is has the word hydro then an element name ending in –ic and the word acid, it is binary. It contains H+ cation and a nonmetal with it’s normal charge for the anion. Yes, you must still balance charges. Hydrosulfuric acid = H+ and S2- = H2S If it does not have the word hydro at the beginning, then it is H+ and a polatomic ion. If the root word ends in –ic followed by the word acid, then the polyatomic ion ends in – ate. Ex. Sulfuric acid – must have come from H+ and the sulfate ion (SO42-). Therefore the formula for sulfuric acid is H2SO4, remember to balance your charges. If there is no hydro and the acid’s root name ends in –ous, then the polyatomic ion in the acid ends in –ite. Ex. Sulfurous Acid – must have come from H+ and the sulfite ion (SO32-). Therefore the formula for sulfurous acid is H2SO3. Review Practice Write the formula for the following compounds: 1) Sodium nitrite - _________________________ 26) Carbon disulfide - _______________________ 2) Sodium carbonate - ______________________ 27) Iron (II) sulfate - ________________________ 3) Disulfur dichloride - _____________________ 28) Iron (III) phosphate - _____________________ 4) Sodium sulfate - ________________________ 29) Acetic acid - ____________________________ 5) Nitrogen triflouride - _____________________ 30) Iron (III) bromide - ______________________ 6) Potassium hydroxide - ____________________ 31) Carbon dioxide - ________________________ 7) Potassium nitrate - _______________________ 32) Strontium chloride - _____________________ 8) Potassium sulfite - _______________________ 33) Strontium hydroxide - ____________________ 9) Carbonic acid - _________________________ 34) Strontium nitrate - _______________________ 10) Potassium phosphate - ___________________ 35) Dichlorine heptoxide - ____________________ 11) Cadmium oxalate - ______________________ 36) Phosphoric acid - ________________________ 12) Hydrochloric acid - _____________________ 37) Strontium sulfite - _______________________ 13) Diphosphorous trioxide - _________________ 38) Phosphorous acid - ______________________ 14) Cadmium carbonate - ____________________ 39) Strontium sulfide - ______________________ 15) Cadmium sulfate - ______________________ 40) Iron (III) sulfate - _______________________ 16) Carbon tetrabromide - ___________________ 41) Iron (III) phosphate - ____________________ 17) Cadmium phosphate - ___________________ 42) Mercury (II) bromide - ___________________ 18) Aluminum bromide - ____________________ 43) Mercury (II) carbonate - __________________ 19) Aluminum nitrate - _____________________ 44) Mercury (II) sulfide - ____________________ 20) Nitric acid - ___________________________ 45) Dinitrogen tetroxide - ____________________ 21) Aluminum nitrate - _____________________ 46) Bismuth (III) telluride - __________________ 22) Iron (II) oxide - ________________________ 47) Nickel (II) fluoride - _____________________ 23) Iron (II) hydroxide - _____________________ 48) Cobalt (III) oxide - ______________________ 24) Carbon monoxide - _____________________ 49) Zinc cyanide - __________________________ 25) Iron (II) carbonate - _____________________ 50) Silver perchlorate - ______________________