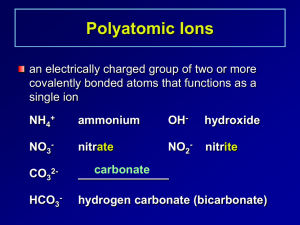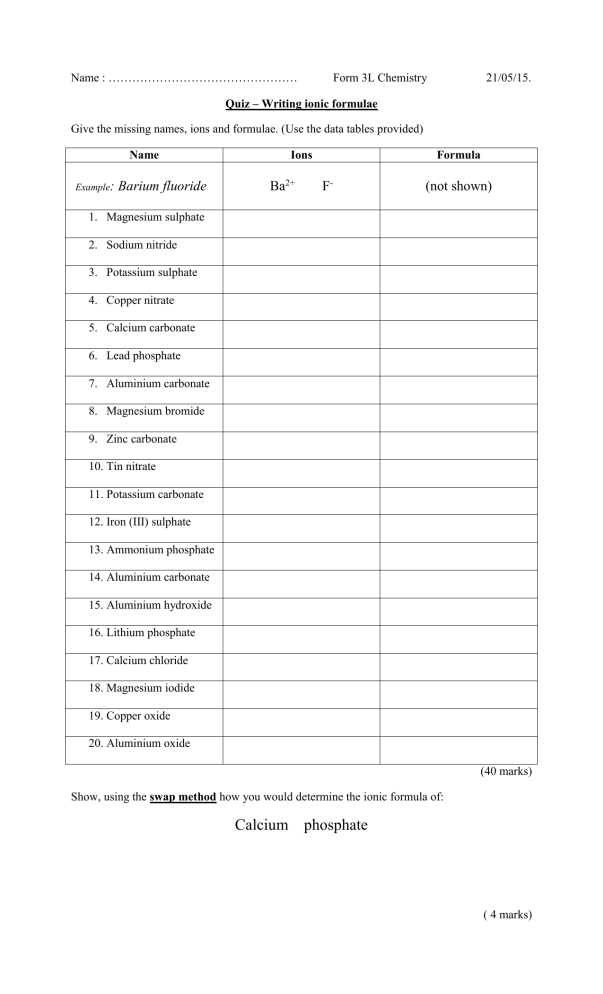
Name : ………………………………………… Form 3L Chemistry 21/05/15. Quiz – Writing ionic formulae Give the missing names, ions and formulae. (Use the data tables provided) Name Example: Barium fluoride Ions Ba2+ Formula F- (not shown) 1. Magnesium sulphate 2. Sodium nitride 3. Potassium sulphate 4. Copper nitrate 5. Calcium carbonate 6. Lead phosphate 7. Aluminium carbonate 8. Magnesium bromide 9. Zinc carbonate 10. Tin nitrate 11. Potassium carbonate 12. Iron (III) sulphate 13. Ammonium phosphate 14. Aluminium carbonate 15. Aluminium hydroxide 16. Lithium phosphate 17. Calcium chloride 18. Magnesium iodide 19. Copper oxide 20. Aluminium oxide (40 marks) Show, using the swap method how you would determine the ionic formula of: Calcium phosphate ( 4 marks) (20 marks) Total 64 marks.