9.2 Answer Key
advertisement

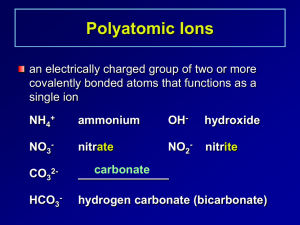
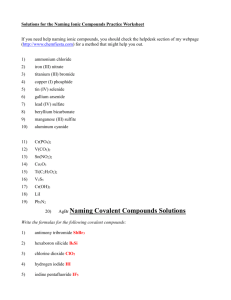
HW 9.2 #14,15,17,18,47,49,52,53 14. Binary ionic compounds (compounds composed of 2 elements) are written using the name of the cation followed by the name of the anion. 15. To write the formula of a binary ionic compound, write the symbol of the cation followed by the symbol of the anion. Use subscripts to determine the appropriate ratio of the cations to anions such that the overall charge of the compound is zero. 17. a. BeCl2 b. Cs2S c. NaI d. SrO 18. a. Cr(NO2)3 b. NaClO4 c. Mg(HCO3)2 d. Ca(C2H3O2)2 47. All compounds are electrically neutral, so they have zero charge. 49. To determine the charge of a transition metal cation (or any variable charge ion) you must look at the overall charge of the anion and mathematically calculate the positive charge needed to make the compounds electrically neutral. 52. Parentheses are used when more than a single polyatomic ion is needed to balance the formula. 53. NH4 + Sn4+ NO3NH4NO3 Ammonium nitrate Sn(NO3)4 Tin(IV) nitrate Fe3+ Fe(NO3)3 Iron(III) nitrate Mg2+ Mg(NO3)2 Magnesium nitrate CO32(NH4)2CO3 Ammonium carbonate Sn(CO3)2 Tin(IV) carbonate Fe2(CO3)3 Iron(III) carbonate MgCO3 Magnesium carbonate CNNH4CN Ammonium cyanide Sn(CN)4 Tin(IV) cyanide Fe(CN)3 Iron(III) cyanide Mg(CN)2 Magnesium cyanide PO43(NH4)3PO4 Ammonium phosphate Sn3(PO4)4 Tin(IV) phosphate FePO4 Iron(III) phosphate Mg3(PO4)2 Magnesium phosphate