Compound Naming Race
advertisement

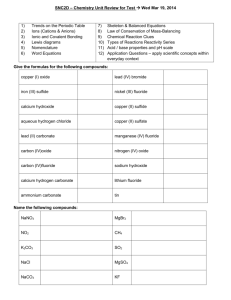
Name _______________ Compound Naming Practice Remember, ionic compounds have NO prefixes, so the lack of a prefix does NOT mean that it contains just one of those atoms. Review Section 6.3 for naming conventions. Important Notes: (1) the Roman numerals next to the transition metals denote the size of the positive ionic charge and (2) you need to use the polyatomic ions table if the anion does not end in “ide” 1) iron (III) phosphide __________________ 2) cobalt (III) carbonate __________________ 3) lithium oxide __________________ 4) aluminum carbonate __________________ 5) nickel (III) sulfite __________________ 6) potassium oxide __________________ 7) manganese (IV) carbonate __________________ 8) lithium arsenide __________________ 9) chromium (VI) sulfate __________________ 10) calcium bromide __________________ 11) sodium nitrate __________________ 12) cobalt (III) sulfide __________________ 13) iron (II) sulfite __________________ 14) vanadium (V) phosphate __________________