Gas Laws
advertisement

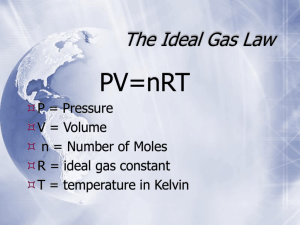
Pressure Volume Moles Temperature Common units Atmospheres (atm) mm Hg Torr kiloPascals (kPa) Pounds per square inch (psi) Bar or millibar (b or mb) Influences Weight of air or water above you Force caused by gas particle collisions with the container wall 1 atm 760 torr 760 mmHg 101.325 kPa 14.69 psi Typically in Liters mL = cc or cm3 Gas laws are independent of mass, but are dependent on moles, which represents number of particles Exception is Graham’s Law Must use Kelvins K = oC + 273.16, but we can get away with saying 273 PV/nT = PV/nT Cross out any factors that are either constant or unmentioned Use this only if a factor is changing Boyles Law PV Charles’ Law VT Avogadro’s Law Vn Gay-Lussac: PT PV=nRT Use when you have all factors but one and the system is not changing R = ideal gas constant Values for R: depends upon your unit of pressure 0.08206 L atm/mol K 62.36 L mmHg/mol K 62.36 L torr / mol K 8.314 L kPa/mol K Pressure Tot = S partial pressure Mole fraction = pressure fraction Pressure Tot = P coll gas + P water vapor Total Pressure = atmospheric pressure Water vapor pressure can be looked up in a table by temperature Pressure of dry gas = Atmospheric pressure – water vapor pressure Have a mixture of 2 mol He, 4 mol Kr, and 4 mol Ar. If the total pressure is 760 mmHg, what is the partial pressure of Ar? Mole fraction of Ar = 4/(2+4+4) or .4 Partial pressure of Ar = 760 x .4 Partial pressure of Ar = 304 mmHg Two gases in a mixture are at the same temperature Same temp means same avg kinetic energy ½ mv2 = ½ mv2 The lower mass will have the higher velocity A lower mass gas will diffuse or effuse faster May have to use PV=nRT to find moles of known or unknown Be careful with your phase symbols. ALL gases must be considered