Mixed PV = nRT problems
advertisement

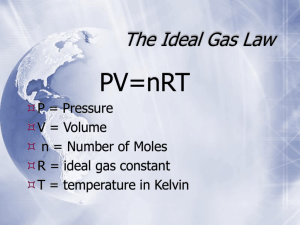
Mixed PV = nRT problems Name ______________________________ If the pressure is given in atmospheres (atm), the R value will be .0821 L atm/ mol °K If the pressure is given in mm Hg, the R value is 62.4 L mm Hg/ mol °K If the pressure is given in kPa, the R value is 8.314 L kPa/ mol °K 1. If the temperature is 25.0 degrees C, and the pressure is 0.800 atm, how many moles of gas are contained in 1.25 L of gas? 2. Find the mass of 5.60 liters of oxygen gas (O2) at 1.00 atm and 297.5 K. 3. Calculate the volume of 4.00 grams of O2 gas at 23.5 degrees C and 83.0 kPa. 4. Determine the mass of 3550 mL of NH3 gas at 0.921 atm and 27.0 degrees C 5. A balloon is filled with 0.159 moles of He and is tied down outside so it wont float away. If the air pressure is 755 mm Hg and the balloon has a volume of 4.00 liters, what is the outside air temperature? (Assume the He inside the balloon is at the same temperature as the outside air.) (R = 62.4 L mm Hg/ mol K) 6. A child receives a balloon filled with 2.30 L of helium from a vendor at an amusement park. The temperature outside is 311 K. The pressure is 1.00 atm. What will the volume of the balloon be (in liters)when the child brings it home to an air-conditioned house at 295 K? Assume the pressure stays the same. Answers to mixed PV=nRT 1. 2. 3. 4. 5. 6. 4.09 X 10-2 mol gas 7.34 g O2 3.71 L O2 2.26 g NH3 31.4 °C 2.18 L He