The Ideal Gas Law
advertisement
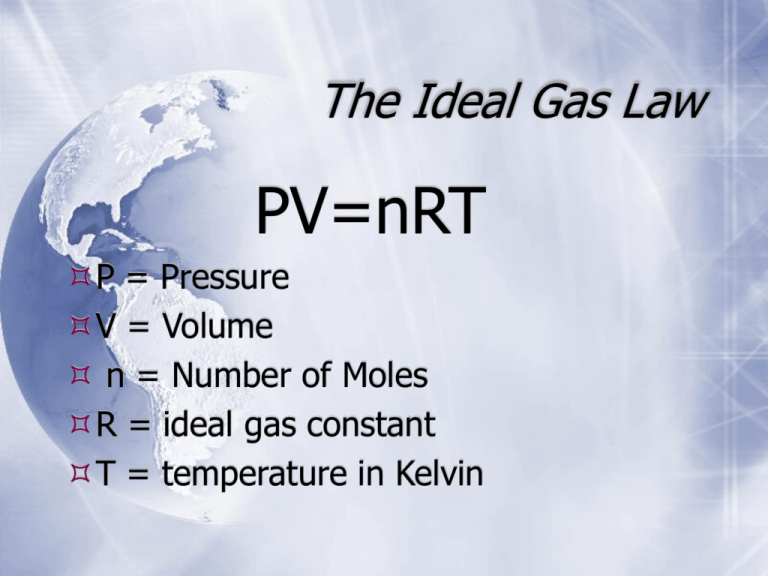
The Ideal Gas Law PV=nRT P = Pressure V = Volume n = Number of Moles R = ideal gas constant T = temperature in Kelvin QuickTime™ and a Sorenson Video 3 decompressor are needed to see this picture. R -The ideal gas constant Depends on unit of pressure 0.0821 L . Atm / K . mol 62.4 L . mmHg / K . mol 8.31 L . kPa / K . mol Ideal Gas Law example problem Calculate the pressure of 1.65 g of helium gas at 16.0oC and occupying a volume of 3.25 L? You will need g to moles and Celsius to Kelvin: 1.65 g He 1 mole He 4.0 g He = 0.412 mol He K = oC + 273 ; 16. 0 + 273 = 289 K For this problem you will need to pick an R value. For this problem I will choose to use the R value containing kPa. I picked it. You can’t do anything about it. So; just try and stop me. Plug and Chug baby, get ‘R done. Do it. Come on I dare ya. Ideal Gas Law example problem P x 3.25 L = 0.412 mol x 8.31 kPa . L x 289 K mol . K Do the algebra and solve; if you do it right, guess what? You get the answer right. Neat concept, huh? = 304 kPa Your turn How many moles of gas are present in a sample of Argon at 58oC with a volume of 275 mL and a pressure of 0.987 atm. Ideal Gas Law example problem Answer 0.987 atm x 0.275 L = n x 0.0821 L . Atm x 331K mol . K Do the dew; oops, I mean the algebra and presto; the answer with the correct number of sig figs is.. Do you know how to keep a so called chem student in suspense? Do ya? = 0.00999 mol Ar Congrats - you can plug and chug. Bye Bye now.











