IDEAL GAS LAW
advertisement

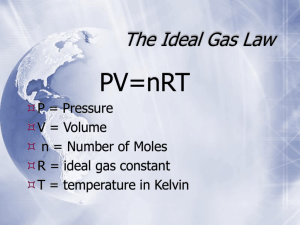
IDEAL GAS LAW A. Expresses a mathematical relationship between P, T, V, and moles of a gas. 1. Formula: PV = nRT n = moles of the gas R = ideal gas constant = 8.314 L-kPa mol-K or 0.0821 L-atm mol-K 2. These values for R are derived from STP. You can relate any of the other gas calculations to this formula in order to solve for the unknown. 3. Can be used to evaluate the behavior of a gas sample, find density of a gas at non-STP conditions, or solve a gas stoichiometry at non-STP conditions. B. Examples: 1. A student collects 425 ml of oxygen at 24oC and a pressure of 0.899 atm. How many moles of oxygen did the student collect? PV = nRT (0.899)(.425) = n(0.0821)(297) n = 0.0157 mol oxygen 2. Determine the molar mass of an unknown gas that has a volume of 72.5 ml, temperature of 68oC, and a pressure of 0.980 atm. The mass of the gas sample is 0.207 g. PV = nRT (0.980)(.0725) = n (0.0821)(341) n = 0.00254 mol molar mass = 0.207 g/0.00254 mol = 81.5 g/mol C. Practice 1. Calculate the molar mass of a gas if 35.44 g of the gas stored in a 7.50 L tank exerts a pressure of 99.5 kPa at a constant temperature of 35.5 °C. 2. Find the volume of 2.40 mol of gas whose temperature is 50.0 °C and whose pressure is 2.00 atm.