PPT 2 - Teach.Chem
advertisement

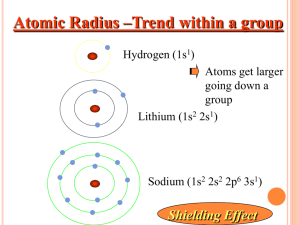
r dr Approximate bonding atomic radii for the elements have been tabulated. The distance between bonded nuclei can be approximated by adding radii from both atoms. e.g., Bonding atomic radii are as follows: C = 0.77 A, Br = 1.14 A So the approximate distance between bonded C and Br nuclei = 0.77 + 1.14 = 1.91 A Atomic Radius As we go down a group, atomic radius… increases. -- principal quantum number increases (i.e., a new energy level is added) As we go from left to right across the Table, atomic radius… decreases. -- effective nuclear charge increases, but principal quantum number is constant more p+, but no new (i.e., farther away) energy levels Coulombic attraction depends on… amount of charge 2+ 2– 1+ 1– distance between charges 2+ 2+ – + H He ++ + + – – – – 2– 2– As we go , more coulombic attraction, no new energy level, more pull, smaller size Arrange the following atoms in order of increasing atomic radius: Sr, Ba, Cs Sr < Ba < Cs Ionization Energy: the minimum energy needed to remove an e– from an atom or ion M(g) + 1st I.E. M+(g) + e– M+(g) + 2nd I.E. M2+(g) + e– M2+(g) + 3rd I.E. M3+(g) + e– Successive ionization energies are larger than previous ones. -- (+) attractive force remains the same, but there is less e–/e– repulsion The ionization energy increases sharply when we try to remove an inner-shell electron. e.g., For Mg, 1st IE = 738 kJ/mol 2nd IE = 1,450 kJ/mol 3rd IE = 7,730 kJ/mol (strong evidence that only valence e– are involved in bonding) As we go down a group, 1st IE… decreases. -- more e–/e– repulsion and more shielding Generally, as we go from left to right, 1st IE… Exceptions: Be: 1s2 2s2 B: 1s2 2s2 2p1 e.g., B < Be 2p B doesn’t like Subshells prefer to be either completely filled OR half-filled. (easier to remove B’s single 2p e– than one of Be’s two 2s e–s) …than any of these. N: 1s2 2s2 2p3 More stable to have O: 1s2 2s2 2p4 than to have 2p This e– is easier to remove… First down a group… Electron affinity: the energy change that occurs when an e– is added to a gaseous atom For most atoms, adding an e– causes energy to be… released. eq. for e– affinity: A + e– A– Exceptions: noble gases: the added e– must go into a new, higher energy level group 2 metals: the added e– must go into a higher-energy p orbital group 15 elements: the added e– is the first one to double-up a p orbital The halogens have the most (–) electron affinities, meaning that they become very stable when they accept electrons. O F more (–) more willing to –141 –328 = – e affinity accept an e– S Cl –200 –349 Electron affinities don’t vary Se Br much going down a group. –195 –325 Te I –190 –295 He + Ne + Ar + Kr + Xe +