Final Exam Chem-225 1435-1436
advertisement

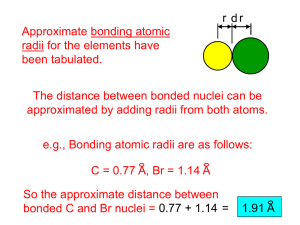
المملكة العربية السعودية وزارة التعليم العالي جامعة المجمعة قسم الحاسب Kingdom of Saudi Arabia Ministry of Higher Education Majmaah University Computer Science Dept. قسم الحاسب:المستوى هـ1436/3/15 :تاريخ االمتحان CHEM-225 :رقم ورمز المادة الكيمياء العامة:اسم المادة :الرقم الجامعي Answer the following questions: Q1. Put circle around the correct answer of the following: Elements are composed of extremely small particles called: (a) atoms (b) molecules (c) ions (d) compounds According to Dalton, all matters consists of atoms which: (a) extremely small particles (b) indivisible particles (c) invisible particle (d) all The subatomic particles which repel each other are (a) electrons (b) protons (c) neutrons (d) answers a and b Atomic number is: (a) the number of electrons (b) the number of neutron in the nucleus (c) the number of protons in the (d) answers a and c are correct nucleus Electron configuration of sodium (atomic number=11) is: (a) 1s2 1p2 2s22p5 (b) 1s2 2s22p53s2 2 2 6 1 (c) 1s 2s 2p 3s (d) 1s1 2s32p63s1 Electron configuration of nitrogen (atomic number=7) is: (a) 1s2 2s22p3 (b) 1s2 2s22p13s2 2 2 2 1 (c) 1s 2s 2p 3s (d) 1s2 2s12p13s3 Covalent bond is formed between: (a) Two non-metals (c) metal and nonmetals (b) Two metals (d) all Ionic bond is formed between: (a) Two non-metals (c) metal and nonmetals (b) Two metals (d) all :اسم الطالب Polar covalent bond such as: (a) Cl-Cl (c) MgCl2 (b) H-Cl (d) NaCl Non-polar covalent bond such as: (a) Br-Br (c) MgCl2 (b) H-Br (d) NaCl Q2. Solve of the following problems: 1. Calculate the number of moles in 3.01 × 1020 molecules of sulfur dioxide (SO2). Answer: ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 2. A certain substance is found to contain 59.9% oxygen and 40.1% sulfur by mass. What is the empirical formula of this substance? (atomic weight for O = 16 , S = 32). Answer: -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 3. A 250-ml flask, open to the atmosphere, contains 0.011 mol of air at 0 °C. Upon heating, part of the air escapes; how much remains in the flask at 100°C? Answer: ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 4. Calculate the mass percent of sodium chloride in a solution prepared by dissolving 24 g of NaCl in 152 g of water. Answer: 5. -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------5. Calculate the concentration of mercury as parts per million (ppm) in a solution of 5 x 10-4 mg of mercury per 1 g water. Answer: ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------6. Calculate the pH and pOH of an HCl solution which has a [H+] = 1.0 x 10-3 Answer: ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 7. Determine the equilibrium constant value (Kc) value for the following reaction , if the concentration of each compound at equilibrium given as: N2(g) + 3H2(g) ↔ 2NH3(g) at 500 K [N2] = 3.0 x 10-2 M [H2] = 3.7 x 10-2 M [NH3] = 1.6 x 10-2 M Answer: -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 8. Calculate Kp value For the following reaction: N2(g) + 3H2(g) ↔ 2NH3(g) at 400 K If the value of Kc was found to be 0.500 Answer: ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------Q3-Complete the following Element /Molecule Oxidation no. of Al in Al2O3 Oxidation no. of C in CCl4 Oxidation no. of S in Na2SO4 Oxidation number Class of chemical reaction Example Decomposition reaction Single replacement reaction Double replacement reaction Combination reaction 2Na(s) + 2H2O (L) Cu(s) → --------------------------------------------- + FeSO4 (aq) → ---------------------------------------------- Cl2(aq) + 2NaBr(aq) → ---------------------------------------------Good Luck