PHY4605 Problem Set 6 Solutions
advertisement

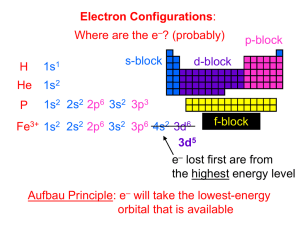
1 PHY4605 Problem Set 6 Solutions 1. Atomic configurations. Griffiths Problem 5.12. (a) H: 1s; He: 1s2 ; Li: 1s2 2s; Be: 1s2 2s2 ; B: 1s2 2s2 2p; C: 1s2 2s2 2p2 ; N: 1s2 2s2 2p3 ; O: 1s2 2s2 2p4 ; F: 1s2 2s2 2p5 ; Ne: 1s2 2s2 2p6 (b) H: 2 S1/2 ; He: 1 S0 ; Li: 2 S1/2 . There is only one possibility in each of these cases for the term symbol, because ` = 0, so the triangle rule allows only one value for j, i.e. s. For the other cases one must be careful to determine first the possible total `, then the possible total s, then the possible j’s which can result. B: the one extra p electron means ` = 1, s = 1/2, so the possibilities for j are 1/2, 3/2: 2 P1/2 , 2 P3/2 . C: two 2p electrons can have spins which add to 1 or 0, while their orbital a.m. can be 2, 1, or 0. This makes ten possible combinations: 1 S0 , 3 S1 , 1 P1 , 3 P0 , 3 P1 , 3 P2 , 1 D2 , 3 D1 , 3 D2 , 3 D3 . For N there are 3 ` = 1 momenta which can add to 0,1,2, or 3, and 3 spins 1/2 which can add to 1/2, or 3/2: 4 S3/2 , 2 S1/2 ,4 P1/2 , 4 P3/2 , 4 P5/2 , 2 P1/2 , 2 P3/2 , 4 D1/2 ,4 D3/2 , 4 D5/2 ,4 D7/2 , 2 D3/2 , 2 D5/2 , 4 F3/2 ,4 F5/2 ,4 F7/2 ,4 F9/2 , 2 F7/2 ,2 F5/2 . 2. Yukawa Hydrogen Griffiths Problem 7.14. q Take φ = πb13 e−r/b , b a variational parameter. The expectation value of the kinetic energy hT i has the same form as for usual Hydrogen, but with a0 → b, i.e. hT i → ~2 . 2mb2 The expectation value of the Yukawa potential in φ is hV i = = = ≈ Z −µr e2 4π ∞ 2 −2r/b e − dr r e 4π²0 πb3 0 r Z ∞ 2 e 4 =− dr r e−(µ+2/b)r 4π²0 b3 0 e2 4 1 e2 1 − = − 4π²0 b3 (µ + 2/b)2 4π²0 b(1 + µb/2)2 2 e 1 − (1 − µb + 3µ2 b2 /4), 4π²0 b 2 where the last expansion follows for sufficiently small µ. The expectation value of H is then ~2 e2 − (1 − µb + 3(µb)2 /4)/b 2 2mb 4π²0 µ ¶ 1 ∂hHi ~2 e2 2 =0 = − 3 + − 3µ /4 ∂b mb 4π²0 b2 hHi = ⇒ This is a cubic equation, but we only need the perturbative solution. The leading order solution, if we neglect the µ2 term, gives b ≈ 4π²0 ~2 = a0 , me2 so write b = a0 + δ and expand for small δ: µ ¶ 1 1 1 2 0 = − + − 3µ /4 (a0 + δ)3 a0 (a0 + δ)2 µ2 1 3 δ −3 (a + ...) ⇒ 0 ≈ −1 + 1 + a0 4 a0 0 δ ≈ 3µ2 a20 /4 a0 So b ≈ a0 (1 + 3µ2 a20 /4). Inserting into the expression for hHi, we find hHi = −R + e2 µ/(4π²0 ) + O(µ2 ). 3. Rubber band Helium Griffiths Problem 7.17. √ √ (a) First invert variable change, r1 = (u + v)/ 2; r2 = (u − v)/ 2; ´ ³ 2 ³ 2 2 ∂ f r12 + r22 = u2 + v 2 . Now I want to show that ∂∂xf2 + ∂∂xf2 = ∂u 2 + 1 2 x ∂2f ∂vx2 ´ , also for 3 y and z. Explicitly, ¶ µ ∂ 2f ∂f ∂ux ∂f ∂ux 1 ∂f ∂f = + =√ + ∂x1 ∂ux ∂x1 ∂vx ∂x1 2 ∂ux ∂vx µ ¶ ∂ 2f ∂f ∂ux ∂f ∂ux 1 ∂f ∂f = + =√ − ∂x2 ∂ux ∂x2 ∂vx ∂x2 2 ∂ux ∂vx µ ¶ ∂ 2f 1 ∂ ∂f ∂f =√ + ∂x21 2 ∂x1 ∂ux ∂vx µ 2 ¶ 1 ∂ 2 f ∂vx ∂ 2 f ∂ux ∂ 2 f ∂vx ∂ f ∂ux = √ + + + 2 ∂vx ∂x1 2 ∂u2x ∂x1 ∂ux ∂vx ∂x1 ∂vx ∂ux ∂x1 µ 2 ¶ 2 2 ∂ f ∂ f 1 ∂ f +2 + 2 = 2 2 ∂ux ∂ux ∂vx ∂vx µ ¶ 2 ∂ f 1 ∂ ∂f ∂f =√ − 2 ∂x2 2 ∂x2 ∂ux ∂vx ¶ µ 2 ∂ 2 f ∂vx ∂ 2 f ∂ux ∂ 2 f ∂vx 1 ∂ f ∂ux = √ + − + 2 ∂vx ∂x2 2 ∂u2x ∂x2 ∂ux ∂vx ∂x2 ∂vx ∂ux ∂x2 µ 2 ¶ 1 ∂ f ∂ 2f ∂ 2f = + − 2 . 2 ∂u2x ∂ux ∂vx ∂vx2 Adding the two results for the two second derivatives gives the desired result. As a consequence, we may write ~2 1 λ H = − (∇2u + ∇2v ) + mω 2 (u2 + v 2 ) − mω 2 2v 2 2 ¶ µ 4 µ 2m 2 ¶ 2 ~ 1 ~ 1 2 2 2 2 2 2 = − ∇ + mω u + − ∇ + (1 − λ)mω v 2m u 2 2m v 2 which is a sum of 3D SHO’s if λ < 1! (b) Since the system separates into two SHO’s, we know the exact energies. In √ particular the exact ground state energy is (3/2)~ω(1 + 1 − λ). (c) The properly normalized ground state wavefunction of the 1D SHO is 2 /2~ ψ0 (x) = (mω/π~)1/4 e−mωx (mω/π~)3/4 e−mωr 2 /2~ (Griffiths 2.59), so the 3D SHO ground state is . The problem asks us simply to evaluate the energy in the product state of two such oscillators, without the λ term (in Sec. 7.2 the product of hydrogenic orbitals is the ground state only if the electron-electron 2 2 interaction is neglected). So we take ψ(r1 , r2 ) = (mω/π~)3/4 e−mω(r1 +r2 )/2~ , and calculate the expectation value of the energy 3 3 λ ~ω + ~ω − h mω 2 |r1 − r2 |2 i 2 2 4 ³ ´3 Z λ λ 2 2 2 2 2 mω −h mω |r1 − r2 | i = − mω e−mω(r1 +r2 )/2~ (r1 − r2 )2 d3 r1 d3 r2 . 4 4 π~ hHi = 4 Now note (r1 − r2 )2 = r12 + r22 − 2r1 · r2 . The r1 · r2 term vanishes by symmetry (the integrand for fixed r2 is odd in each component of r1 ), and the r12 and r22 terms contribute the same, so ³ ´3 Z λ λ 2 2 2 2 2 mω −h mω |r1 − r2 | i = − mω 2 e−mω(r1 +r2 )/2~ r12 d3 r1 d3 r2 4 4 π~ Z ∞ Z ∞ ³ mω ´3 λ 2 2 2 −mωr12 /2~ 4 (4π) = − mω e r1 dr1 e−mωr2 /2~ r22 dr2 2 π~ 0 0 " #" µ # r ¶2 r 4 5 ~ 8m ω 1 ~ π~ 3 π~ 3 = −λ = − λ~ω π~3 4 mω mω 8 mω mω 4 So hHi = 3~ω(1 − λ/4), which must be greater than or equal to exact energy for 0 ≤ λ ≤ 1. By expanding the square root in the exact expression (answer to b)), you can convince yourself this is indeed the case.