17 GROUP BONDING the HALOGENS
advertisement

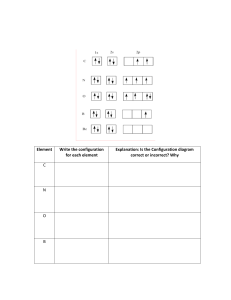
GROUP 17 BONDING the HALOGENS Bond mainly by ionic bonding to become 1- ions with noble gas configurations. The ions are isoelectronic to the noble gas of the SAME period, which is the kernel of that element. High electronegativity and High ionization energy causes an electron to be gained, completing the p sublevel and the outer shell octet. As you read down the group, electronegativity, ionization energy and nonmetal reactivity DECREASE, atomic radius INCREASES. See concept map. All negative ions are larger than the neutral atom from which they are derived, due to a gained electron. EXAMPLES The configuration of Neon 1s2↑↓ The configuration of FLOURINE (ground) 1s2↑↓ 2s2 ↑↓ 2p6 ↑↓ ↑↓ ↑↓ 2s2 ↑↓ 2p5 ↑↓ ↑↓ ↑_ The configuration of FLOURIDE ION [F]1- 1s2↑↓ 2s2 ↑↓ 2p6 ↑↓ ↑↓ ↑↓ AFFINITY and REDUCTION The addition of an electron is called ELECTRON AFFINITY, the energy that is evolved to do so is called AFFINITY ENERGY. This process is exothermic – energy releasing, the energy must have a sink that will absorb it.. In the diagram below, the following is important: 1. The electron gained will most likely be obtained by from a metal. 2. The resulting ion is isoelectronic to the noble gas of the same period. 3. AFFINITY ENERGY is released. F + 1e- The electron is from another atom 1s2↑↓ 2s2 ↑↓ 2p5 ↑↓ ↑↓ ↑ + 1e- F[Ne]1s2↑↓ 2s2 ↑↓ 2p6 ↑↓ ↑↓ ↑↓