Empirical Formula vs. Molecular Formula
advertisement
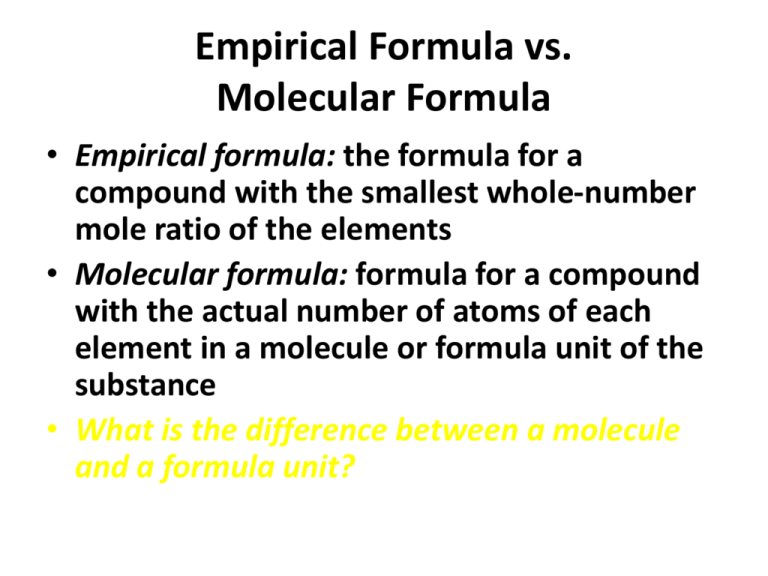
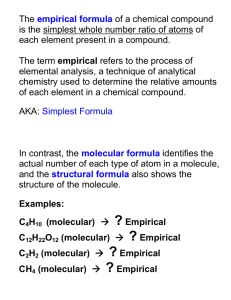
Empirical Formula vs. Molecular Formula • Empirical formula: the formula for a compound with the smallest whole-number mole ratio of the elements • Molecular formula: formula for a compound with the actual number of atoms of each element in a molecule or formula unit of the substance • What is the difference between a molecule and a formula unit? Empirical Formula vs. Molecular Formula, continued…… • Example of the difference: Benzene is composed of 6 carbon and 6 hydrogen (C6H6). • What is the ratio of carbon-to-hydrogen in benzene? • If we reduce the compound formula to its “smallest whole-number mole ratio of the elements” (empirical formula), we are left with CH • Acetylene’s molecular formula is C2H2. What is its empirical formula? Calculating Empirical Formula From Percent Composition 1. If given percent composition of a compound, make the percent of each element that element’s mass in grams, so the whole compound has a molar mass of 100 grams. 2. Convert the mass of each element to moles. 3. If the answers (moles) are not whole numbers, divide all answers by the smallest number of moles present. 4. If still not whole numbers, multiply all answers by the smallest factor that will give you whole numbers. Calculating Empirical Formula From Percent Composition, continued….. • Examples: P.349 (144, 147) • Practice Problems (p.333) --- reference Example Problem on p.332 for needed guidance