Formula & Percent Composition Packet
advertisement
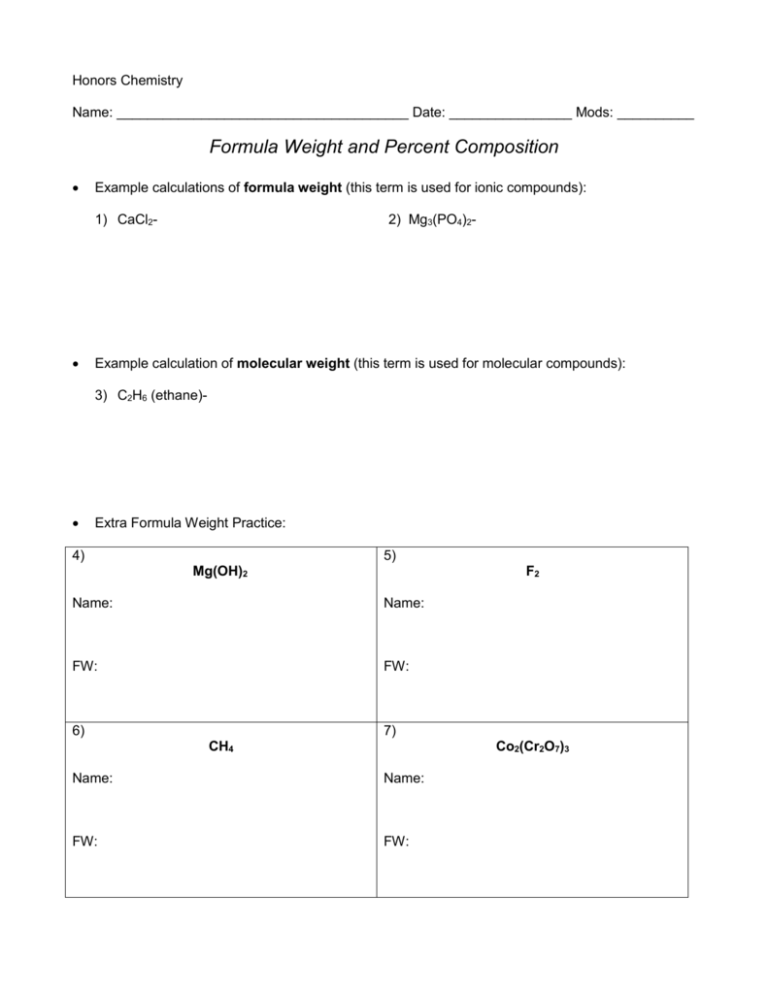
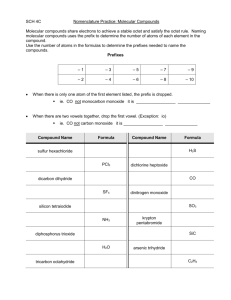
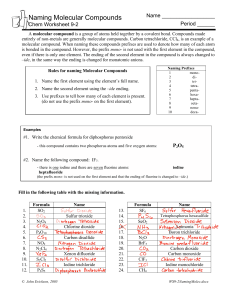
Honors Chemistry Name: ______________________________________ Date: ________________ Mods: __________ Formula Weight and Percent Composition Example calculations of formula weight (this term is used for ionic compounds): 1) CaCl2- 2) Mg3(PO4)2- Example calculation of molecular weight (this term is used for molecular compounds): 3) C2H6 (ethane)- Extra Formula Weight Practice: 4) 5) Mg(OH)2 F2 Name: Name: FW: FW: 6) 7) CH4 Co2(Cr2O7)3 Name: Name: FW: FW: Percent Composition: In-Class Examples The percent by mass of an element in a compound is the number of grams of the element divided by the number of grams of the compound, multiplied by 100. Think “part” over “whole”. % by mass A= (atomic mass of element A) (# of atoms of element A) x 100 Formula weight of whole compound 1) Magnesium nitrate % Mg: _____________ 2) Formula: _______________________ % N: _____________ Tetrasulfur decaoxide Formula: _______________________ % S: _____________ 3) Aluminum dichromate % Al: _____________ 4) Chloric acid % O: _____________ % O: _____________ Formula: _______________________ % Cr: _____________ % O: _____________ Formula: _______________________ How much chlorine can be recovered from 175 grams of chloric acid? Formula and Molecular Weights Practice Directions: Determine the formula weight of each of the following compounds: 1. N2O5 Name: ________________________________________ 2. FeCO3 Name: ________________________________________ 3. Ca(C2H3O2)2 Name: ________________________________________ 4. (NH4)3PO3 Name: ________________________________________ 5. sodium nitrite Formula: ______________________________________ 6. copper (II) thiocyanate Formula: ______________________________________ 7. disilicon hexabromide Formula: ______________________________________ Percentage Composition Worksheet Give the % composition of all elements in these compounds. Show ALL work! 1) ammonium sulfite % N = __________ Formula: ___________________ % H = __________ 2) aluminum acetate % Al = __________ % S = __________ % O = __________ Formula: ____________________ % C = __________ 3) beryllium nitride %H = __________ % O = __________ Formula: ____________________ % Be = __________ 4) copper (II) hydroxide % Cu = __________ % N = __________ Formula: ____________________ % O = __________ % H = __________ Tougher Percent Composition Problems 1) How much iron can be recovered from 25.0 g of iron (III) oxide? 2) How much phosphorus can be produced from 125.0 g of diphosphorus pentoxide? 3) How much oxygen would be found in 48.4 g of chlorous acid?