Aldehydes vs. Ketones
advertisement

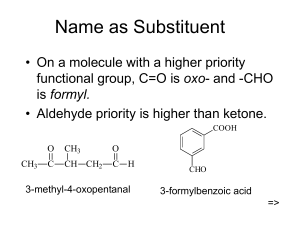
ALDEHYDES VS. KETONES Concept Presentation by: Maninder Kaur & Shirley Easo. CONTENTS: Introduction to Aldehydes and Ketones. Aldehydes & ketones: poisons, flavors & perfumes Nomenclature for aldehydes & ketones Preparation of aldehydes & ketones The polarity of the carbonyl group Some members of the series Distinguishing aldehydes & ketones Aldehydes & ketones : applications References. BIG IDEAS Organic compounds have predictable chemical and physical properties determined by their respective structures. Organic compounds can be synthesized by living things or through artificial processes. Organic chemical reactions and their applications have significant implications for society, human health, and the environment. OVERALL EXPECTATIONS B1. assess the social and environmental impact of organic compounds used in everyday life, and propose a course of action to reduce the use of compounds that are harmful to human health and the environment; B2. investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds; B3. demonstrate an understanding of the structure, properties, and chemical behavior of compounds Within each class of organic compounds AT THE END OF THIS UNIT, STUDENTS SHOULD BE ABLE TO: Compare & contrast aldehydes & ketones Analyze, understand and justify why aldehydes are more reactive than ketones Design reactions for obtaining simple aldehydes and ketones Describe the occurrence of aldehydes and ketones in nature and give examples of the applications of them. ALDEHYDES & KETONES. For centuries we have used bloodhounds to locate missing persons or criminals. Ever wondered why this works? Its because of the amazing ability of bloodhounds to detect scent molecules, and because individuals have characteristic odor prints that are as unique as their fingerprints or DNA. This scent is due to Aldehydes and ketones. These are Compounds containing carbonyl group, mostly used for fragrance and flavor. MINDS ON: Students are made aware of the importance of carbonyl compounds by connecting it to simple house hold items they can relate to. Items like solvents, nail polish remover, perfumes etc. are brought into the classroom The students in groups will think pair & share and record on chart paper the uses of the products, advantages/disadvantages for the environment and society, hazards and creating a model of the compounds using tooth picks and marshmallows (or other materials). NOMENCLATURE: Students are then introduced to the nomenclature using molecular models and interesting fun you tube clips: It’s a Family Thing: http://www.youtube.com/watch?v=mAjrnZ-znkY ALDEHYDES AND KETONES In an aldehyde, an H atom is attached to a carbonyl group O carbonyl group CH3-C-H In a ketone, two carbon groups are attached to a carbonyl group O carbonyl group CH3-C-CH3 NAMING ALDEHYDES IUPAC -Replace the -e in the alkane name with-al Common -Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O O O H-C-H CH3-C-H CH3CH2C-H methanal ethanal propanal (formaldehyde) (acetaldehyde) (propionaldehyde) NAMING KETONES In the IUPAC name, the -e in the alkane name is replaced with -one In the common name, add the word ketone after naming the alkyl groups attached to the carbonyl group O O CH3 -C-CH3 CH3-C-CH2-CH3 Propanone 2-Butanone (Dimethyl ketone) (Ethyl methyl ketone) FLAVORS OF ALDEHYDES AND KETONES: O O CH CH O CH=CH CH HO OCH 3 Benzaldehyde (almonds) Vanillin (vanilla beans) Cinnamaldehyde (cinnamon) OO Butter flavor Clove flavor CH3-C-C-CH3 butanedione O CH3-C-CH2CH2CH2CH2CH3 2-heptanone -SOME ANTS USE A KETONE CALLED 2HEPTANONE TO WARN OF DANGER. a group of complex ketones, called pheromones, are used by many insects and animals as sex attracters. USUALLY USED TO PRESERVE BIOLOGICAL SPECIMENS. FORMALDEHYDE ; AN ALDEHYDE WITH A NOT SO PLEASANT SMELL IS USED. SPECIMENS ARE PICKLED IN FORMALDEHYDE. THE POLARITY OF CARBONYL COMPOUNDS: A KEY CONCEPT Carbonyl compounds are polar, containing a dipole along the carbon-oxygen double bond with no hydrogen bonding. Inquiry based learning incorporating role play and openedded meaningful questions. ROLE PLAY Role play: Student A : Oxygen Student B : Carbon Poles/sticks: Double bond (electrons) 2 Paper crowns with ᵟ+ and ᵟ-signs Students A and B can role play the polarity of the carbon and oxygen double bond by using two long sticks/poles. The student representing oxygen (A), (being more electronegative) will be pushing the sticks more towards himself/herself. While the student representing B will be letting the sticks move more towards student A. When the sticks (representing electrons) move more towards student A, students will be then asked open ended & meaningful questions. Students will be then asked where should the ᵟ+ and ᵟ– charged crowns be placed. . Student A representing OXYGEN:ELECTROPHILLIC :loves electrons: so the shared electrons are displaced more towards oxygen, making it partially electronegative : ᵟ– crown is therefore placed on student A. An eager volunteer from class comes and brings a paper crown with ᵟ– sign and places on student A (Oxygen). Similarly a paper crown with ᵟ+ charge is placed on student B (Carbon) HOW TO PREPARE SUCH INTERESTING COMPOUNDS: STUDENTS WOULD BE TAUGHT ABOUT THIS CONCEPT INCORPORATING JIGSAW/MIND MAPS AND/OR SMART BOARD . When a primary alcohol is oxidized, an H atom remains on the C atom, and an aldehyde is produced. Example: Ethanol (primary alcohol) + (O) → ethanal (aldehyde) + water HOW TO PREPARE SUCH INTERESTING COMPOUNDS: When a secondary alcohol is oxidized, the carbonyl group formed is attached to two alkyl groups, forming a ketone. Example: 2-propanol (secondary alcohol) + (O) → propanone (ketone) + water When a tertiary alcohol is oxidized, no oxidation occurs because there are no available hydrogen atoms on the central C atom. Therefore there is basically no reaction DISTINGUISHING ALDEHYDES & KETONES (INQUIRY BASED LEARNING) Problem statement: What effect does bulkiness of the alkyl groups (steric hindrance) have on the reactivity of ketones when you compare with aldehydes? Aldehydes vs. Ketones: Reactivity Generally, aldehydes are more reactive than ketones. There is a combination of steric hindrance and inductive effects that make ketones react slower than the corresponding aldehydes. Thus aldehydes are more reactive than ketones because : Bulky alkyl groups present in ketones makes it difficult (hinder) the action of nucleophiles Alkyl substituents are also weakly electron donating thus they make the carbon in the carbonyl less electrophilic. MINDS ON: “STERIC HINDRANCE IS LIKE A FAT GOALIE” http://masterorganicchemistry.com/2011/07/ 18/steric-hindrance-is-like-a-fat-goalie/ Quiet reading time : Students read the article “Sportswriter Todd Gallagher asked a question that every hockey fan has probably asked at one point. “Could a morbidly obese goaltender succeed in the NHL?” . ROLE PLAY: STUDENTS ROLE PLAY STERIC HINDRANCE A group of volunteers (alkyl groups) surround a parcel (carbon atom) and try to block a lone student (reactant) trying to approach or open the parcel. The reactant will be trying to react (open/recover) with the carbon atom (parcel) .But the group of volunteers acting like alkyl groups(like the ones in ketones) will try to hinder(block) the reaction -Steric hindrance is the big barrier for chemical reactions.* Remove it, and speed up the reaction. STERIC HINDRANCE TESTS TO INQUIRE IF THE GIVEN MATERIAL IS KETONE OR ALDEHYDE: Minds ON + Hands lab Students watch a u tube clip on tollen’s (silver mirror) test and perform the corresponding lab. http://www.youtube.com/watch?v=zFMsqcGdZCc &feature=related http://www.youtube.com/watch?v=GkyxNpitgeE 1. USING TOLLENS' REAGENT (THE SILVER MIRROR TEST) . To carry out the test, you add a few drops of the aldehyde or ketone to the freshly prepared Tollems’ reagent (caution while handling silver nitrate), and warm gently in a hot water bath for a few minutes. INFERENCES: Ketones: No change in the colourless solution. Aldehydes: The colourless solution produces a grey precipitate of silver, or a silver mirror on the test tube. Aldehydes reduce the diamminesilver(I) ion to metallic silver. Because the solution is alkaline, the aldehyde itself is oxidised to a salt of the corresponding carboxylic acid. http://www.youtube.com/watch?v=zFMsqcGdZCc &feature=related SUGGESTED PROJECTS: “The smell of formaldehyde was once common in the hallways of high schools, as it was used a preservative of biological specimens. This use has largely been discontinued.” Choose an aldehyde or ketone and do an extensive investigation to study the properties, benefits, environmental effects etc. (Students will work in small groups of 2-3 and do an extensive study on the properties/advantaged/disadvantages of a carbonyl compound of their choice. They will be allowed to present the study in differentiated ways. APPLICATION/ASSESSMENT: Some questions to guide their investigation : Why did you choose this particular compound? Is your molecule an aldehyde or ketone? Give reason why? Does your molecule contain any other functional groups? List and show where they are in the molecule. APPLICATION/ASSESSMENT: What are the main properties and important uses of the chosen compound? What are the environmental/social advantages & disadvantages of the compound? Are there any suggestions how you can use this compound in environmental friendly ways Suggested carbonyl compounds: butyraldehyde, vanillin, acetophenone, cinnamaldehyde, camphor, pyrethrin, carvone, muscone, acetone, formaldehyde, acetaldehyde, diethyl ketone, etc. APPLICATION /ASSESSMENT In cases of severe diabetes, a patient’s tissues cannot break down glucose, and, instead, the body breaks down fat for its energy. The fats are broken down in the liver and muscles, producing several compounds called “ketone bodies,” one of which is acetone. a. The acetone produced in this process is carried in the blood and urine. Explain why acetone is soluble in these aqueous solutions. . b. When fats are the main source of energy production, there is an overproduction of ketone bodies, leading to a condition called ketosis. A patient with untreated diabetes may have a blood concentration of acetone of 20 mg/ 100 mL.Convert this concentration to mol/L. . c. Acetone is volatile and is exhaled with the breath. Suggest a reason why, like untreated diabetic patients, people who are severely starved or dieting may have a smell of acetone on their breath – a diagnostic symptom of ketosis. d. Other ketone bodies lower blood pH, causing a condition called acidosis, which can lead to coma and death. Research the symptoms and effects of ketosis and acidosis and how these conditions may be avoided. . Present your project to the class in the form of any differentiated activity , role play, PowerPoint presentation, Bristol board presentation, etc. Also include a molecular model of the compounds. DIFFERENTIATED LEARNING, ACCOMMODATION AND INTEGRATION: Resource: ”Start where they are” Karen Hume (1, 2, 3) Pre-assess students one - two weeks before the beginning of the unit. Teacher should use the observations from the handson activities as a form of individual pre-assessment. Teacher should regroup students for the next activities based on these results to make students engage with the essential concepts of the unit. Teacher should discuss the intended learning outcomes with the students at the beginning of the unit. . Tiered assignments will be given to make sure students get the big ideas and the essential skills involved and the assignments will be given at different levels of complexity and open-endedness. For example students who already understood the concept of steric hindrance are given a virtual lab assignment in which they must develop and test hypotheses related to the topic, while other students are given more direct instruction on the concept. Students are given a choice that contains a list of possible activities they can complete to learn more about aldehydes & ketones. DIFFERENTIATED LEARNING, ACCOMMODATION AND INTEGRATION: Accommodation: ELL-students: 1. Students use computer to type their exit tickets, quiz. 2. Teacher – student conference/ interview to assess the student learning and understanding of the concepts HAZARDS AND SAFETY PRECAUTIONS: Acetaldehyde is harmful by inhalation, ingestion and through skin absorption. Some experiments with animals suggest that this substance may be anticipated to be a carcinogen. Formaldehyde 37% solution causes burns. Very toxic by inhalation, ingestion and through skin absorption. Readily absorbed through skin. Probable human carcinogen. Mutagen. May cause damage to kidneys, allergic reactions, sensitization and heritable genetic damage HAZARDS AND SAFETY PRECAUTIONS: Sulfurous acid is harmful if swallowed. Corrosive - causes burns. Skin or eye contact will lead to severe irritation or burns Safety glasses, protective gloves, good ventilation. The experiments should be performed under a portable fume hood! REFERENCES: http://wwwpersonal.une.edu.au/~sglover/CHEM110TopicI/sl d001.htm http://www.uniregensburg.de/Fakultaeten/nat_Fak_IV/Organisc he_Chemie/Didaktik/Keusch/D-Aldehyde-e.htm http://www.youtube.com/watch?v=zFMsqcGdZCc &feature=related http://www.youtube.com/watch?v=GkyxNpitgeE http://ltp.learnetic.com/index.php/resources/ald ehydes_ketones_nomenclature_boiling_point_sol ubility_occurrence.html REFERENCES: New sites http://www.wiziq.com/tutorial/108521aldehydes-and-ketones http://www.ccwcs.org/content/teachingguided-inquiry-organic-chemistry-labs http://quizlet.com/6635254/ahs-sch4uorganic-flash-cards/ REFERENCES: Davies, L., Di Giusepp, M., Jenkins, F., Plumb, D., and van Kessel, H. (2003). Nelson Chemistry 12. Nelson Education Ltd. Toronto, Canada. p. 38-57. Davies, L., Di Giusepp, M., Jenkins, F., Plumb, D., and van Kessel, H. (2003). Nelson Chemistry 12. Nelson Education Ltd. Toronto, Canada. p. 38-57.