Name as Substituent

Name as Substituent
• On a molecule with a higher priority functional group, C=O is oxo - and -CHO is formyl.
• Aldehyde priority is higher than ketone.
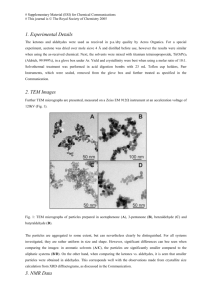
COOH
CH
3
O CH
3
C CH CH
2
O
C H
CHO
3-methyl-4-oxopentanal 3-formylbenzoic acid
=>
4.5 Isomerism in aldehydes and ketones
• Aldehydes and ketones are constitutional isomers
• Aldehydes and ketones can have skeletal and positional isomers if there are enough carbons.
• Stereoisomers are also possible if there is a ring or C=C in the molecule
4.6 Selected Common aldehydes and ketones
• Formaldehyde
• Acetone
• Vanillin
• Benzaldehyde
• Cinnamaldehyde
• Butanedione
Formaldehyde
• Gas at room temperature.
• Formalin is a 40% aqueous solution.
H
C
O
H
H
C
O
H
O
C
H
H trioxane, m.p. 62
C heat
O
H
2
O
H C H formaldehyde, b.p. -21
C
HO
OH
H C
H formalin
=>
Industrial Importance
• Acetone and methyl ethyl ketone are important solvents.
• Formaldehyde used in polymers like
Bakelite
.
• Flavorings and additives like vanilla, cinnamon, artificial butter.
=>
• Formaldehyde -formalin
• Acetone – solvent and metabolic product
• Vanillin - vanilla flavoring
• Benzaldehyde - almond flavor
• Cinnamaldehyde - cinnamon
• Butanedione - butter
4.7 Physical properties
• Boiling points – page 121
• Solubility – water solubility page 123
Boiling Points
• More polar, so higher boiling point than comparable alkane or ether.
• Cannot H-bond to each other, so lower boiling point than comparable alcohol.
=>
Solubility
• Good solvent for alcohols.
• Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from
O-H or N-H.
• Acetone and acetaldehyde are miscible in water.
=>
4.8 Preparation of Aldehydes and
Ketones
• Oxidation
– 2 alcohol + Na
2
Cr
2
O
7
– 1 alcohol
aldehyde
ketone