Molecular shapes_1551_VB
advertisement

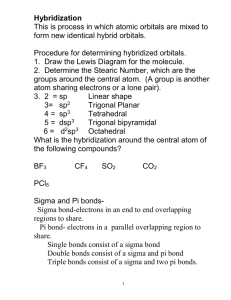
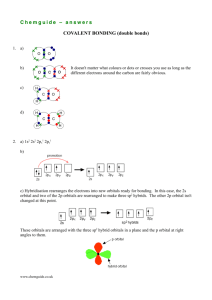
Valence bond theory Electrons are not simply dots And bonds are not sticks Learning objectives Describe principles of valence bond theory Predict hybridization of orbitals based on Lewis dot structures and electronic geometry Describe difference between sigma and pi bonding Taking it to the next level: acknowledging orbitals VSEPR is quite successful in predicting molecular shapes based on the simplistic Lewis dot approach But our understanding of the atom has the electrons occupying atomic orbitals How do we reconcile the observed shapes of molecules with the atomic orbital picture of atoms Valence bond theory Valence bond theory is the simplest approach to an orbital picture of covalent bonds Each covalent bond is formed by an overlap of atomic orbitals from each atom The individual orbital identity is retained The bond strength is proportional to the amount of orbital overlap Overlap of two 1s orbitals in H2 Overlap of two 2p orbitals directed along the bond axis (sigma bond) Overlap of p and s orbitals Problems with tetrahedral bonds In CH4 the bonds are all equivalent and at angles of 109.5° The 2p orbitals in C are at 90° - far from optimum for overlap The ground state configuration is 2s22p2 Reconcile these facts with the known structure Hybridization The wave mechanics permits mixing of the atomic orbital set to produce “hybrid” orbitals Hybridization alters the shape and energy of the original In the case of C, the differences between the 2s and 2p are smoothed out and a homogeneous collection of four sp3 hybrid orbitals is produced sp3 hybridization Formally, one of the 2s electrons is promoted to the empty 2p orbital (an energy cost, which is repaid on bond formation) The four basis orbitals are then “hybridized” to yield the set of four sp3 Tetrahedral directions and sp3 hybrids Valence bond picture of CH4 Each C sp3 hybrid contains one electron Each H 1s contains one electron Lone pairs occupy sp3 hybrid orbitals Valence bond picture of the tetrahedral electronic geometry provides same results for the molecules with lone pairs Notes on hybridization The total number of orbitals is unchanged Four atomic orbitals (s + 3 x p) give four hybrid orbitals (4 x sp3) The electron capacity remains unchanged There is one hybridization scheme for each of the five electronic geometries The same hybridization scheme is always used for a given electronic geometry sp hybridization for linear geometry One s and one p orbital sp2 hybridization for trigonal planar One s and two p orbitals Sigma and pi bonding The hybridized orbitals describe the electronic geometry: bonds along the internuclear axes (sigma bonds) The “unused” p orbitals overlap in a parallel arrangement above and below the internuclear axis (pi bonds) Comparison of pi and sigma bonding Pi bonding accounts for bond multiplicity Two unused p orbitals in sp hybrid (linear geometry) Two pi bonds N≡N triple bond (one sigma, two pi) One unused p orbital in sp2 hybrid (trigonal planar geometry One pi bond C=C double bond (one sigma, one pi) Valence bond picture of ethylene H2C=CH2 Sigma bonds between C and H (blue/red) and C and C (blue) Six electrons around C Pi bond between C and C (green) Two electrons around C Valence bond picture of acetylene HC≡CH Sigma bonds between C and H (red and blue) and C and C (blue) 4 electrons around C Two pi bonds between C and C (green) 4 electrons around C Beyond coordination number 4 Invoke empty d orbitals (impossible for second row elements) One d orbital for trigonal bipyramidal Two d orbitals for octahedral Number of orbitals in hybrid always equals number of charge clouds Trigonal bipyramid – sp3d Octahedral –sp3d2 Shortcomings of valence bond The orbitals still maintain atomic identity Bonds are limited to two atoms Cannot accommodate the concept of delocalized electrons – bonds covering more than two atoms Problems with magnetic and spectroscopic properties