Ketones - Bryan, Rau, Tellier
advertisement

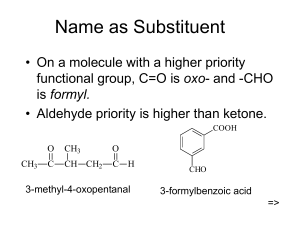
KETONES MIKAYLA BRYAN, JOSH RAU, AND LOGAN TELLIER MR. SNYDER M O N D A Y D E C E M B E R 2 ND, 2 0 1 3 . WHAT IS TO COME… • Structure and Functional Group • Reactions: Oxidation and Hydrogenation • Physical and Chemical Properties • Uses of Ketones • Steps of Ketone Nomenclature and Line Drawings • Examples THE FUNCTIONAL GROUP Characterized by oxygen double bonded to Carbon Carbonyl Group STRUCTURE • Similar to Aldehyde. • Carbonyl group must have two carbons on either side (or other groups possibly). • Must be attached to at least 2nd carbon. ALDEHYDES VS. KETONES Aldehyde -al Ketone -one KETONES • Name was derived from German spelling of a common ketone molecule: acetone. Aketone Ketone **Simplest Form of Ketone 2-propanone REACTIONS Ketone Reactions • The main difference between aldehydes and ketones is a presence of a hydrogen atom. • Historically, the term “oxidation” was used to describe any reaction involving oxygen. • When a secondary alcohol is oxidized, the carbonyl group is formed necessarily attached to two alkyl groups, forming a ketone. • Ketones don’t further into making more compounds once they have been oxidized by the secondary alcohol. • Ketones in general are hormones. • Oxidation reactions for ketones usually involve an acid catalyst. Hydrogenation • A reaction called “hydrogenation” involves a hydrogen gas and a platinum catalyst which produces alcohols. • The process is commonly employed to reduce or saturate organic compounds. • Catalysts are required for the reaction to be usable. • Hydrogenation reduces double and triple bonds in hydrocarbons. APPLICATIONS OF KETONES • Acetone has many uses in industries due to many unique attributes; it is one of the few organic compounds that is infinitely soluble in water and can dissolve organic compounds • Acetone is largely used as an industrial solvent and is often used to dissolve paints, varnishes, resins or nail polish • The low boiling point and volatility of Acetone allows for it to be removed by evapouration when no longer wanted • Methyl Ethyl Ketone or Butanone, like Acetone finds much of its use in industrial applications • MEK is also used as an industrial solvent and is applied in the manufacturing of paraffin wax or textiles • Some other common uses of MEK are in dry erase markers as the solvent of the dye or as ‘modeling glue’ as it dissolves polystyrene • Cyclohexanone is another ketone prominent in industry and manufacturing • Much of the world’s supply of Cyclohexanone is used to produce Nylon as well as to produce Adipic Acid; which is also mainly used in the production of Nylon KETONES IN THE HUMAN BODY • Ketones are also present in the human body in the form of three different ‘Ketone bodies’ • These are produced by the liver from fatty acids to use as energy instead of glucose in times of low food intake • Ketone bodies are largely produced in a state of ketosis; caused by a diet with no carbohydrates and therefore a deficit of glucose in the body Fatty acids • Type 1 diabetics may have such high levels of Ketone bodies in their systems due to poor metabolization of glucose that it will lower the pH of their blood that the kidneys will attempt to excrete all Ketones and glucose from the body, potentially causing deadly dehydration • Ketone bodies are applied in the human bodie under normal circumstances as well, serving as a constant source of energy for vital organs such as the heart and brain. • On top of being involved in hormones, ketones are also prominent in health and diet supplements NOMENCLATURE • REMEMBER: the carbonyl group must be attached to at least the second carbon of the chain. • Ending of ketones is “-one” IUPAC NAMING STEPS 1) Identify longest parent chain and give it an alkane name except replace the -e ending with –one. (Ex. Butane Butanone) 2) Give the position of the carbonyl group before the parent chain name. (Start at the end closest to the carbonyl group. IUPAC NAMING STEPS 3) Name all alkyl or other groups normally and place at beginning of the name (prefix). 4) Put the prefix and suffix together: alkyl groups + parent alkane (position # -one) EXAMPLES 2-propanone or acetone EXAMPLES MULTIPLE CARBONYL GROUPS 2,3,4-pentanetrione EXAMPLES: ALKYL GROUPS • 3-methyl-2-pentanone EXAMPLES: CYCLOALKANE KETONES Cycloheptanone PLEASE NOTE Double or triple bonds: Number the carbons based on the closest carbonyl group The double and triple bonds are numbered through an alkene and alkyne naming system but numbered AFTER carbonyl group EXAMPLES: ALKENES WITH KETONES 3-methyl-4-hexen-2-one EXAMPLES: ALKYNES WITH KETONES 4-octyn-3-one NAME THIS 2,4-diethylcyclohexanone DRAW THIS 3,5-dimethyl-2-hexanone NAME THIS 1,1-dibromo-3-methyl-5-octyn-2-one DIY • Please complete numbers 1-3 in the nomenclature section. • ALSO complete numbers 7-9 in the line drawing section • (Answers will be gone through on white board.) QUESTIONS?? • Please ask any question pertinent to this presentation at this point in time.