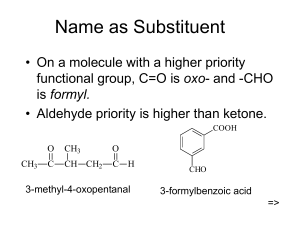
ALDEHYDES AND KEYTONES Experiment # 5 Group # 2 Jamina Fay Sanoy Jasper Marion Ancheta Kevin Gattoc Hans Ruar Joanna Pangilinan Joy Santos Department of Chemistry College of Art and Science, Central Luzon State University October 13, 2021 _____________________________________________________________________________________ ABSTRACT- This study focused on aldehydes and ketones. Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. Ketones contain the carbonyl group bonded to two carbon atoms. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The result showed that the tests performed were capable of detecting the presence or absence of specific functional groups such as aldehydes and ketones. Keywords: Chromic Acid Test, Tollen’s Test, Iodoform Test, 2,4-Dinitrophenylhydrazine (DNPH) Test _____________________________________________________________________________________ INTRODUCTION Aldehydes and ketones contain the carbonyl group. Aldehydes are considered the most important functional group. They are often called the formyl or methanoyl group. Aldehydes derive their name from the dehydration of alcohols. Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. Ketones contain the carbonyl group bonded to two carbon atoms. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen, alkyl or aryl substituents. If at least one of these substituents is hydrogen, the compound is an aldehyde. If neither is hydrogen, the compound is a ketone (Farmer et.al, 2020). METHODOLOGY A. Chromic Acid Test A few drops of formaldehyde, benzaldehyde, cyclohexanone and acetone were placed on four different test tubes. Chromic acid was added to each test tubes and was observed to form a green solution with formaldehyde and benzaldehyde as both were oxidized. No reaction was observed in cyclohexanone and acetone due to the lack of oxidizable hydrogen atom in it. B. Tollen’s Test About 5 drops of formaldehyde, benzaldehyde, cyclohexanone and acetone were placed on four different test tubes. All samples were dissolved using 20 drops diethyl ether. Another 10 drops of tollen’s reagent were added on each test tubes. All test tubes were shaken and placed on a water for a few minutes. After heating the test tubes, a silver mirror precipitate was observed in formaldehyde and benzaldehyde while no silver mirror precipitate was observed in both cyclohexanone and acetone. C. Iodoform Test About 15 drops and more of 6M NaOH was added in formaldehyde, benzaldehyde, cyclohexanone and acetone. All sample were placed on a water bath for 3 minutes. The I2-KI reagent was added in each test tubes for about 20 drops until the color become light brown. Stop adding more of the reagent if there is no changes happen after the addition of 20 drops. No reaction was observed on formaldehyde, benzaldehyde, cyclohexanone. The acetone produced a light-yellow solution with a yellow precipitate. D. 2,4-Dinitrophenylhydrazine (DNPH) Test About 15 drops of 2,4-DNPH was added in test tubes with formaldehyde, benzaldehyde, cyclohexanone and acetone. After which, all samples produce a yellow solution with yellow precipitate in test tube with benzaldehyde. The samples with yellow precipitate were subject to water bath for about 5 minutes and cooled at room temperature. RESULT AND DISCUSSIONS 1. Chemical Tests A. Chromic Acid Test Table 1: Chromic Acid Test Compounds A. Formaldehyde B. Benzaldehyde C. Cyclohexanone D. Acetone Test results Forms green color solution (Aldehydes) Forms green color solution (Aldehydes) No reaction (Ketones) No reaction (Ketones) The table above shows the test result of Chromic acid test for Aldehydes and Ketones. The Formaldehyde forms green solution as it added with Chromic acid it tends to form formic acid and gain 2H atom added with Cr+3 responsible for green solution. The Benzaldehyde also forms green solution as it added with Chromic acid because it oxidized to carboxylic acids while the Cr+6 ion in the chromic acid is reduced to Cr+3. The Cyclohexanone and Acetone forms no reaction because they have no oxidizable Hydrogen atom. B. Tollen’s Test Table 2: Tollen’s Test Compounds A. Formaldehyde B. Benzaldehyde C. Cyclohexanone D. Acetone Test results Forms white precipitate (supposed to form silver mirror) Forms white precipitate (supposed to form silver mirror) No reaction No reaction The table above shows the test result of Tollen’s test for Aldehydes and Ketones. The Formaldehyde and Benzaldehyde precipitates (supposed to form silver mirror). The Cyclohexanone and Acetone gives no reaction because they are not oxidized by Tollens’ reagent. The aldehyde is oxidized by the Tollens reagent and forms a carboxylic acid. The silver ions present in the Tollens reagent are reduced into metallic silver. C. Iodoform Test Table 3: Iodoform Test Compounds A. Formaldehyde B. Benzaldehyde C. Cyclohexanone D. Acetone Test results No changes in color It changes to light brown color It changes to light brown color It changes from clear to translucent appearance Iodoform test helps identify the presence of methyl ketone on the compounds – aldehydes and ketones (Isac-García, Dobado, Calvo-Flores & Martínez-García, 2016). The table above shows the different reaction of the compounds on the iodoform (triiodomethane). Test tube A (which contains Formaldehyde) did not changed to light brown color which indicate that it has no presence of methyl group that is directly attached to a carbonyl. On the other hand, both test tube B (which contains benzaldehyde) and C (cyclohexanone) changed colors to light brown which means that the two compounds are positive to iodoform test. Acetone from the test tube D also changed its appearance but not to light brown, instead it changed from clear to translucent appearance. The test tubes B and C were then combined with sodium hydroxide (NaOH) to turn them back from colorless compounds. Both test tubes did not turn back to colorless compound and stayed in light brown color. D. 2,4-Dinitrophenylhydrazine (DNPH) Test Table 4: 2,4-Dinitrophenylhydrazine (DNPH) Test Compounds A. Formaldehyde B. Benzaldehyde C. Cyclohexanone D. Acetone Test results Forms orange precipitate Forms orange precipitate Forms orange precipitate Forms orange precipitate The table above shows the same results on 2,4-Dinitrophenylhydrazine (DNPH) Test. The four compounds – formaldehyde, benzaldehyde, cyclohexanone, and acetone – formed orange precipitate which indicate the positive test for DNPH test. The melting point of these precipitates is extremely high which support the presence of carbonyl compounds. This is a condensation reaction, which occurs when two molecules join together and lose water (Hill & Holman, 2000). CONCLUSION Four different tests (Chromic acid test, Tollen’s test, Iodoform test, and 2,4Dinitrophenylhydrazine (DNPH) test) were carried out in this laboratory experiment to learn about the chemical properties of Aldehydes and Ketones, as well as to distinguish between the two. The Chromic acid test revealed that Formaldehyde and Benzaldehyde are both Aldehydes, whereas the remaining two samples that were not oxidized were found to be Ketones. In the Tollen’s test, both Formaldehyde and Benzaldehyde formed white precipitate, supposedly silver mirror precipitate, once again indicating that they are Aldehydes. As for the Iodoform test, Benzaldehyde and Cyclohexanone both tested positive, indicating the existence of methyl ketone on the substances. Acetone, on the other hand, switched color from clear to translucent, but not to light brown. Finally, the existence of carbonyl compounds in all of the samples tested was confirmed by the positive results of all of the compounds utilized in the 2,4-Dinitrophenylhydrazine (DNPH) test. However, these tests were not carried out flawlessly, and certain flaws occurred that resulted in unexpected outcomes. During the lab, one issue that was faced was the lack of freshly prepared tollens reagent, or the use of contaminated tollens reagents, which resulted in the creation of white Aldehyde precipitates instead of silver mirror precipitates. Another error was encountered in the Iodoform test, wherein Acetone should have tested positive. As a result of these issues, correct preparation for each tool and sample should be strictly adhered to in order to achieve the intended outcomes. Despite the issues encountered, the results obtained showed that the tests performed were capable of detecting the presence or absence of specific functional groups such as aldehydes and ketones. REFERENCES Benzaldehyde Tests. (1999). Www.Chem.Uiuc.Edu. Retreieved http://www.chem.uiuc.edu/weborganic/orglab/qualknown/Benzaldehyde.htm from: Byjus, (n.d.). General Data Protection Regulation (GDPR) Guidelines BYJU’S. BYJUS. Retrieved October 8, 2021, from https://byjus.com/chemistry/tollens-test/ Farmer (2020). Nomenclature of Aldehydes & Ketones. Chemestry LibreTexts https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Or ganic_Chemistry)/Aldehydes_and_Ketones Graham Hill and John Holman, 2000, adapted from Chemistry in Context, 4th Edition. Joaquín Isac-García, José A. Dobado, Francisco G. Calvo-Flores, Henar Martínez-García (2016), Experimental Organic Chemistry, Academic Press, ISBN 9780128038932, (https://www.sciencedirect.com/science/article/pii/B9780128038932500061) Larsen, D. (2018, June 13). Exceptionally rapid oxime and hydrazone formation promoted by catalytic amine buffers with low toxicity. Chemical Science (RSC Publishing). https://pubs.rsc.org/en/content/articlelanding/2018/sc/c8sc01082j Wellesley Academics (n.d.). Academics.Wellesley. Edu. Chem 211 - Tests for Aldehydes and Ketones. Retrieved October 8, 2021, from http://academics.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/Appendix/Cla ssificationTests/aldehyde_ketone.html