Electron configuration
advertisement

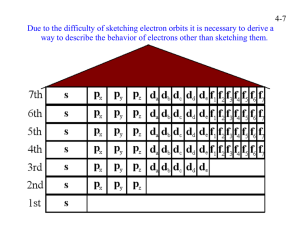
Electrons in Atoms So, you live on Aspen St., but what is your exact address? We know that electrons can occupy energy levels, but they can also reside within sublevels. Each level has a fixed number of sublevels and each sublevel has a fixed number of electrons The orbitals are where an electron spends 90% of its time. Principal Energy Level n=1 n=2 n=3 n=4 Sublevels # of orbitals in each sublevel This shows the different blocks in the Periodic Table. It also shows in what order to write electron configurations (1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d,7p) 1. Aufbau Principle – fill lowest energy levels first 2. Pauli Exclusion Principle – only two electrons in an orbital with opposite spins 3. Hund’s Rule – (bus seat rule) place one electron in each orbital before placing the second H He Be B Kr 4d 5s 4p 3d 4s s 1 2 3p 3s 2p 2s 1s + 1s1 1 2 3 4 5 6 7 p 1 2 3 4 5 6 d 1 2 3 4 5 6 1 2 3 4 5 6 f 7 8 9 10 7 8 9 10 11 12 13 14 That’s right: it goes in the 1s sublevel. And its el. config is 1s2. Notice in the table above where H is – in the area designated as 1s. So where does the next electron go? 4d 5s 4p 3d 4s s 1 2 3p 3s 2p 2s 1s + 1s2 1 2 3 4 5 6 7 p 1 2 3 4 5 6 d 1 2 3 4 5 6 1 2 3 4 5 6 f 7 8 9 10 7 8 9 10 11 12 13 14 If you were thinking it went in the 2s, then you forgot that each orbital can hold up to two electrons. Note how He is right here in the area designated as 1s2 and so its el. config. is 1s2. 4d 5s 4p 3d 4s s 1 2 3p 3s 2p 2s 1s + 1s2 2s2 1 2 3 4 5 6 7 p 1 2 3 4 5 6 d 1 2 3 4 5 6 1 2 3 4 5 6 f 7 8 9 10 7 8 9 10 11 12 13 14 Is this what you were thinking? Good. Now look at the periodic table above, what comes after the 2s sublevel? The 2p sublevel. So what will the next el. conf. be? 4d 5s 4p 3d 4s s 1 2 1 2 3 4 5 6 7 3p 3s 2p 2s 1s + 1s2 2s2 2p1 p 1 2 3 4 5 d 1 2 3 4 5 6 1 2 3 4 5 6 f 7 8 9 10 7 8 9 10 11 12 13 14 Is this what you were thinking? Notice how B is in the 2p1 spot. So its full el. conf. is 1s2 2s2 2p1. What’s next? 6 4d 5s 4p 3d 4s s 1 2 3p 3s 2p 2s 1s + 1 2 3 4 5 6 7 p 1 2 3 4 d 1 2 3 4 5 6 1 2 3 4 5 6 f 7 8 9 10 7 8 9 10 11 12 13 14 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5 6