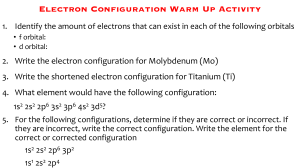
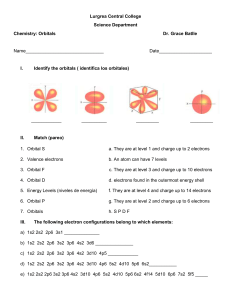
Electron Structure Chemistry Homework
advertisement
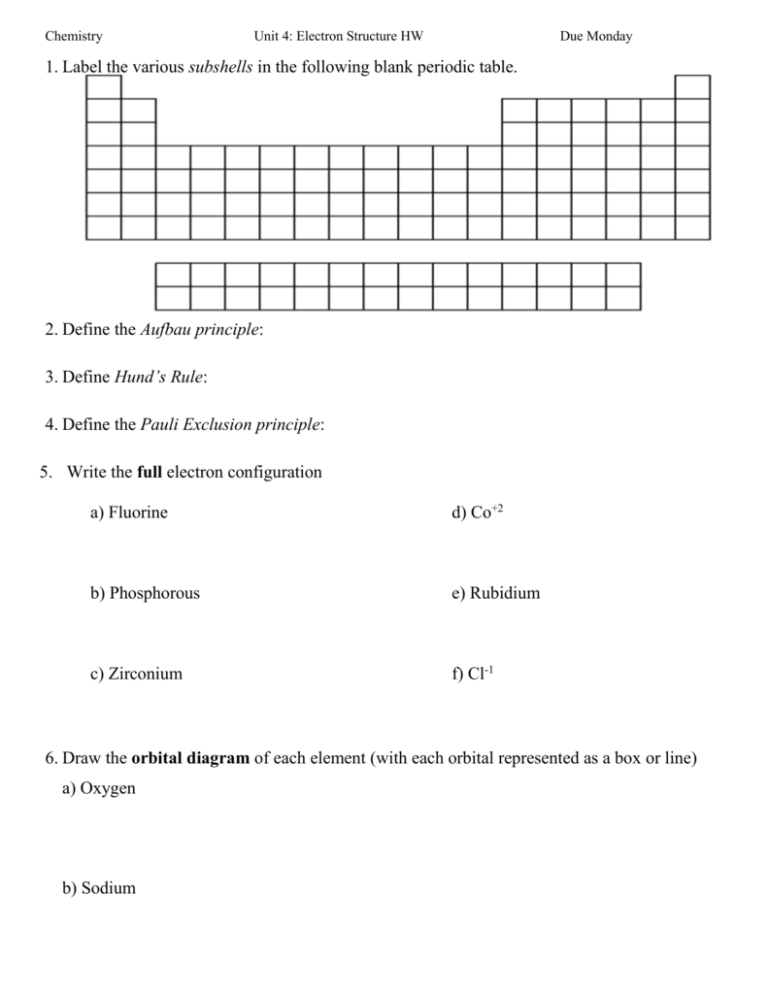
Chemistry Unit 4: Electron Structure HW Due Monday 1. Label the various subshells in the following blank periodic table. 2. Define the Aufbau principle: 3. Define Hund’s Rule: 4. Define the Pauli Exclusion principle: 5. Write the full electron configuration a) Fluorine d) Co+2 b) Phosphorous e) Rubidium c) Zirconium f) Cl-1 6. Draw the orbital diagram of each element (with each orbital represented as a box or line) a) Oxygen b) Sodium Chemistry Unit 4: Electron Structure HW Due Monday c) Argon d) Manganese e) According to (d), is Manganese Paramagnetic or Diamagnetic? 7. Write the electron configuration using the Noble Gas notation a) Oxygen b) Sodium c) Cobalt d) Iodine e) Barium 8. Which elements have the following electron configurations? a) 1s2 2s2 2p6 3s2 3p6 4s2 3d8 b) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 c) [Ar] 4s2 d) [Xe] 6s2 4f14 5d10 6p2 9. What is the significance of valance electrons? 10. Determine the number of valence electrons in the following atoms. a) Ca b) P c) Xe d) Al e) Br 11. Draw the orbital diagram of O-2 and use it to explain why Oxygen forms a -2 charge. (What rule is followed?)