Electron Configuration Practice Worksheet
advertisement

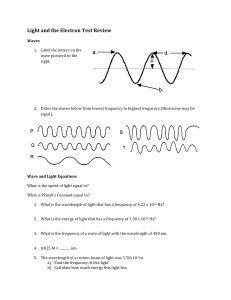
Name___________________________________________ Date_________________ Period______ Electron Configuration Practice Worksheet In the space below, write the ground-state electron configurations of the following elements: (1 point each) 1. sodium ____________________________________________________________ 2. magnesium _________________________________________________________ 3. iron _______________________________________________________________ 4. potassium __________________________________________________________ 5. selenium ___________________________________________________________ In the space below, use noble gas notation to write the ground-state electron configurations of the following elements: (1 point each) 6. cobalt _____________________________________________________________ 7. silver ______________________________________________________________ 8. tellurium ___________________________________________________________ 9. radium ____________________________________________________________ 10. lawrencium ________________________________________________________ Determine what elements are denoted by the following electron configurations: : (1 point each) 11. 1s22s22p63s23p4 ____________________________________________________ 12. 1s22s22p63s23p64s23d104p65s1 ______________________________________ 13. [Kr] 5s24d105p3 ___________________________________________________ 14. [Xe] 6s24f145d6 ___________________________________________________ 15. [Rn] 7s25f11 ______________________________________________________ 1 Name___________________________________________ Date_________________ Period______ Explain what is wrong with the following electron configurations, and then write the electron configuration correctly: (1 point each) 16. 1s22s22p63s23p64s24d104p6 __________________________________________________________________________ __________________________________________________________________________ 17. 1s22s22p63s33d5 __________________________________________________________________________ __________________________________________________________________________ Use the following electron configurations and your periodic table to identify the element: (1 point each) 18. 1s2 2s2 2p6 3s2 3p5 ______________________________________________________ 19. 1s2 2s2 2p6 3s2 3p6 4s2 ___________________________________________________ 20. 1s2 2s2 2p6 3s2 3p6 4s2 3d104p1____________________________________________ 21. Describe the method that you used to solve problems 18-20. Be specific. (3 points) __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ __________________________________________________________________________ 2 Name___________________________________________ Date_________________ Period______ Use the following clues to identify the element. (1 point each) 22. This element has a 3p sublevel that contains 3 electrons. __________________________________________________________________ 23. This element has a 4s sublevel with 2 electrons for its outermost electrons. __________________________________________________________________ 24. This element has 1 electron in its 3d sublevel. __________________________________________________________________ 25. This element has 5 electrons in its 5p sublevel __________________________________________________________________ 27. This element has a completely filled 3p sublevel for its outermost electrons. __________________________________________________________________ Grading scale: 30 points possible A: 27-30 B: 24-26 C: 21-23 D: 18-20 F: <18 Page 1__________ Page 2__________ Page 3__________ Total points____________________ Letter grade____________________ 3