PERIODIC TABLE MEDIA LISTENING GUIDE View the video and
advertisement
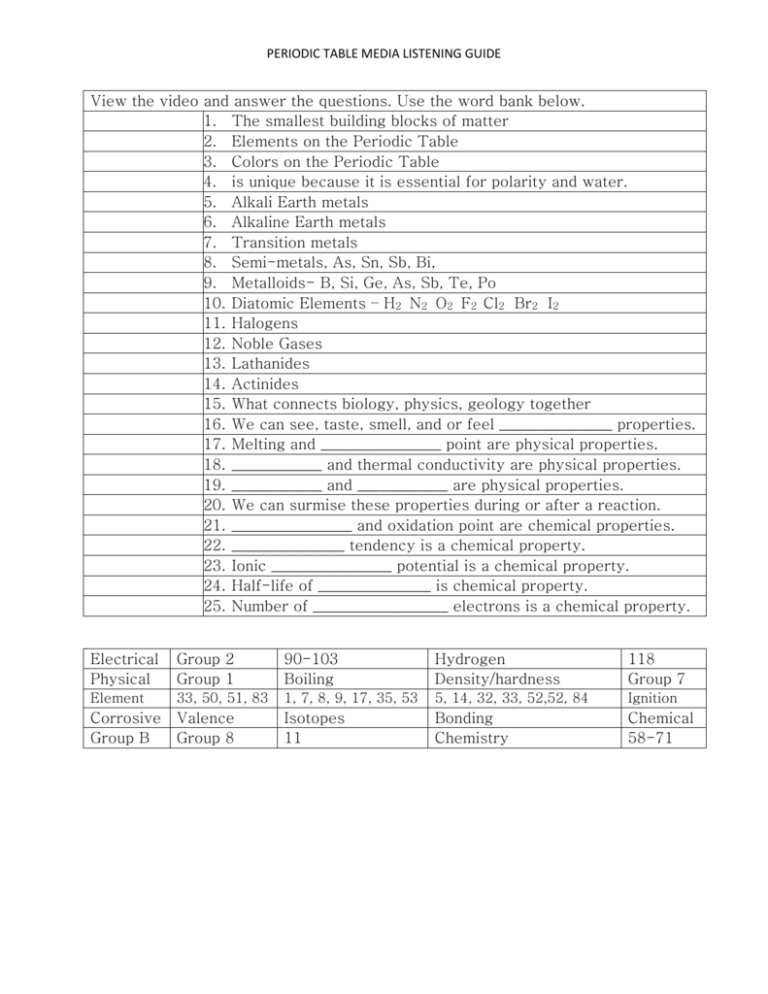
PERIODIC TABLE MEDIA LISTENING GUIDE View the video and answer the questions. Use the word bank below. 1. The smallest building blocks of matter 2. Elements on the Periodic Table 3. Colors on the Periodic Table 4. is unique because it is essential for polarity and water. 5. Alkali Earth metals 6. Alkaline Earth metals 7. Transition metals 8. Semi-metals, As, Sn, Sb, Bi, 9. Metalloids- B, Si, Ge, As, Sb, Te, Po 10. Diatomic Elements – H2 N2 O2 F2 Cl2 Br2 I2 11. Halogens 12. Noble Gases 13. Lathanides 14. Actinides 15. What connects biology, physics, geology together 16. We can see, taste, smell, and or feel _______________ properties. 17. Melting and ________________ point are physical properties. 18. ____________ and thermal conductivity are physical properties. 19. ____________ and ____________ are physical properties. 20. We can surmise these properties during or after a reaction. 21. ________________ and oxidation point are chemical properties. 22. _______________ tendency is a chemical property. 23. Ionic ________________ potential is a chemical property. 24. Half-life of _______________ is chemical property. 25. Number of __________________ electrons is a chemical property. Electrical Physical Group 2 Group 1 90-103 Boiling Hydrogen Density/hardness 118 Group 7 Element 33, 50, 51, 83 1, 7, 8, 9, 17, 35, 53 5, 14, 32, 33, 52,52, 84 Ignition Corrosive Group B Valence Group 8 Isotopes 11 Bonding Chemistry Chemical 58-71 PERIODIC TABLE MEDIA LISTENING GUIDE View the video and answer the questions. Use the word bank below. 26. A. The average atomic weight of all natural isotopes. [always a decimal] b. The number protons and neutrons. [never a decimal] 27. Who originally ordered the first 63 known elements according to atomic weight? 28. Increases as you travel right across a period and up a group 29. Increases as you travel right across a period and up a group 30. Increases as you travel right across a period and up a group 31. Increases as you travel left across a period and down a group 32. Increases as you travel left across a period and down a group 33. The lightest, most abundant element 34. Has the largest atomic radius 35. Has the highest ionization energy 36. Has the strongest electron affinity 37. Is the most metallic element 38. Is the most nonmetallic element 39. The last element in pure stable form 40. Equal the number of protons for a neutral atom 41. Any element above atomic number 91 [Uranium] 42. Who classified the transuranic elements? 43. What organization is charge of the Periodic Table? ◦ 44. Standard temperature and pressure: 1 atm at 273 C 45. Elements with same number of protons but different number of neutrons 46. Heat and _______________ are the only emissions for fuel cell vehicles. 47. 80% of elements are shiny and conduct heat and electricity. 48. 15% of elements are poor conductors of heat and electricity 49. 5% share properties among metals and nonmetals 50. Electrons in the outermost shell determined by group number on periodic table. Atomic Radius Mosley metals Transuranic IUPAC Ionization Energy STP H2O Valence Nonmetallic character Fluorine Fluorine isotopes Atomic weight/ Mass number Hydrogen Seaborg Electron Affinity Francium Electrons Einsteinium Metallic character Mendelev Nonmetals Francium Metalloids