Periodic Table Notes
advertisement

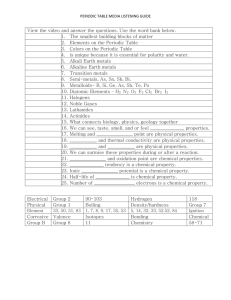
PERIODIC TABLE HISTORY Mid-1800’s 60 elements identified 5 were nonmetal gases @ room temperature. H2, O2, N2, F2, Cl2. Liquids – Hg, Br Rest were solids with varying properties. History cont… Placement of similar property elements. Dimitri Mendeleev – Russian Periodic Table resembles Calendar. What other exhibits of periodicity? Atoms of different elements have different Mass. Atomic weights assigned. History cont… Combining Capacity- 1 of two ways to organize elements. ECl – “Element combining with” Chlorine. 1 to 1 combinations. (KCl, CsCl) MgCl2 – Magnesium Chloride, ECl2 Atomic Number The number of protons in an atom. Distinguishes different elements. Nucleus “Center” of atom that is an area of + positive charge. Along with NEUTRONs…protons make up the MASS NUMBER. Isotopes Atoms of the same atomic number(# of protons), can have different MASS #. They are called ISOTOPES. Atoms of the same element, but with different mass number. Carbon 12 Carbon 14 Table Sections Metals Mettaloids NonMetals Rows & Columns Rows – Periods Columns – Family Hockey Tree Names of Families Alkali Metals Alkalia Earth Metals Transition Metals Boron Group Carbon Group Nitrogen Group Oxygen Group Halogen Group Noble Gases