Physical Science Chpter 4 Study Guide
advertisement

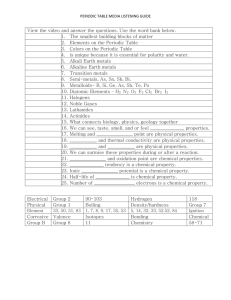
Physical Science Study Guide Chapter 4 Name: ___________________ Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following scientists inferred that an atom’s positive charge must be clustered in the nucleus? a. Niels Bohr b. John Dalton c. Ernest Rutherford d. J.J. Thomson 2. Which particles in atoms have a negative electric charge? a. electrons b. protons c. neutrons d. nuclei 3. Mendeleev created the first periodic table by arranging elements in order of a. decreasing atomic mass. b. increasing atomic mass. c. increasing atomic number. d. increasing melting points and densities. 4. What prediction did Mendeleev make that came true less than 20 years later? a. He predicted the atomic numbers of unknown elements. b. He predicted that a total of 112 elements would be discovered. c. He said that three new elements would be discovered, and he described their properties. d. He said that the periodic table would be developed into 18 families. 5. The elements in a column of the periodic table a. have similar properties. b. are in the same period. c. have the same atomic mass. d. have very similar chemical symbols. 6. Most metals are NOT a. ductile. b. good conductors of heat and electricity. c. liquid at room temperature. d. malleable. 7. In general, which of the following statements about metals is true? a. Metals need to be stored in sealed containers for safety. b. Metals show a wide range of chemical properties. c. Metals are highly reactive substances. d. Metals do not react with oxygen. 8. Which group contains the most elements? a. semimetals b. nonmetals c. metals d. transition elements 9. In the periodic table, the most reactive metals are found a. in Group 1, the first column on the left. b. in Period 1, the first row across the top. c. in Groups 13 through 16 in the center. d. in Periods 6 and 7 at the bottom. 10. Which of the following statements about transition metals is true? a. They are never found uncombined in nature. b. They include familiar metals such as gold, silver, copper, and nickel. c. They are so soft that they can be cut with an ordinary knife. d. They are the most reactive of all the types of metals. 11. Which property of bromine could you NOT predict based on the fact that it is a nonmetal in the halogen family? a. highly reactive b. poor conductor of electricity c. liquid at room temperature d. poor conductor of heat 12. The elements that do not ordinarily form compounds are a. elements in the carbon family. b. metals. c. halogens. d. inert gases. 13. Fluorine, chlorine, bromine, and iodine are part of a family called a. inert gases. b. semimetals. c. halogens. d. alkali metals. 14. Which group of elements shares characteristics with both metals and nonmetals? a. salts b. semimetals c. halogens d. lanthanides 15. In 1896, the French scientist Henri Becquerel discovered a. light-emitting polymers. b. a process to turn natural rubber into a hard, stretchable polymer. c. radioactive decay. d. how to make alloys. 16. During radioactive decay, atomic nuclei of unstable isotopes a. give off nuclear radiation. b. are broken down by radioactive bacteria. c. form chemical bonds. d. are unchanged. 17. A piece of paper will provide protection from a. alpha radiation. b. beta radiation. c. gamma radiation. d. gamma rays. 18. In radiation therapy, a. isotopes are traced through a chemical reaction. b. unhealthy human cells are destroyed. c. radioactive isotopes are used as fuel. d. weak spots in water pipes are found. 19. The most useful property of semimetals is their a. ability to be pulled out into long wires. b. softness and malleability. c. tendency to be unreactive. d. varying ability to conduct electricitic current. 20. Which form of nuclear radiation consists of high-energy waves similar to X-rays? a. alpha particles b. beta particles c. gamma rays d. isotopes Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 21. Protons have no charge; they are neutral. _________________________ 22. The modern periodic table is organized according to atomic mass. _________________________ 23. The horizontal rows in the periodic table are known as groups. _________________________ 24. The elements in a group of the periodic table have similar characteristics. _________________________ 25. Describing a metal as malleable means that it can be pounded into a new shape. _________________________ 26. The most chemically reactive metals are in Group 1 of the periodic table. _________________________ 27. In general, the physical properties of nonmetals are similar to the properties of metals. _________________________ 28. Atoms of the halogen family of elements typically gain two electrons when they react. _________________________ 29. A radioactive isotope that can be followed through the steps of a chemical reaction is a(n) indicator. _________________________ 30. Positively charged particles in an atom’s nucleus are called neutrons. Completion Complete each statement. 31. Mendeleev discovered that periodic patterns appeared when he arranged the elements in order of increasing ____________________. 32. A column of elements in the periodic table is called a group, or ____________________. 33. Most metals are in the ____________________ state at room temperature. 34. Elements with atomic numbers higher than 92 are not found naturally on Earth, so they must be made, or ____________________, by crashing nuclear particles into each other. 35. Elements that form diatomic molecules, or molecules of two atoms each, are commonly found on the ____________________ side of the periodic table. 36. Nonmetals are ____________________ conductors of heat and electricity. 37. At room temperature, all the semimetals are solids, while most nonmetals are ____________________. 38. A substance that will conduct electricity only under certain conditions is called a(n) ____________________. 39. A(n) ____________________ particle is positively charged and consists of two protons and neutrons. 40. The spontaneous emission of radiation by an unstable atomic nucleus was named ____________________ by Marie Curie Short Answer Use the diagram to answer each question. Atoms of Some Common Elements Element Atomic Number Mass Number Protons Neutrons Electrons Sodium 11 ? 11 12 ? Magnesium 12 24 12 ? 12 Aluminum ? 27 13 14 13 Phosphorus 15 31 ? 16 15 41. What is the total number of electrons in an atom of sodium? 42. How many neutrons are in an atom of magnesium? 43. How many protons are in an atom of phosphorus? 44. The element silicon has been omitted from this table. It appears in Period 3 of the periodic table between aluminum and phosphorus. Given that information, which of the five columns in the chart could you fill in for silicon? Use the diagram to answer each question. 45. Which group of elements reacts violently with elements from Group 1? 46. If a metal reacts violently with water, in which group is it likely to be found? 47. What name is given to the elements in Groups 3 through 12? How do their properties tend to compare with the elements to the left and right of these groups? 48. Locate the box in Group 18 in the fourth period. Predict the state of matter and the chemical reactivity of the element that belongs in that box. 49. Most of the elements that form a zigzag line in the periodic table belong to one major group. What is that group, and what kinds of properties do its elements tend to have? 50. What are the two most important alkali metals? Why are they so important?