Ch 5 The Periodic Table
advertisement

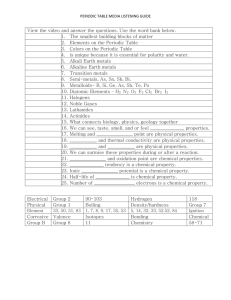
The Periodic Table Study it like the cool kids… Physical Science – SS -- 10/21/15 What do you already know about the periodic table? BONUS: What do you want to know about the Periodic Table? (Go and write it on the GIANT PostIt on the cabinet.) The Search for Order Until 1750, scientists had identified only 17 elements, mainly metals, such as copper and iron. More discovered and the need to order them increased. FIRST ATTEMPT: In 1789, Antoine Lavoisier grouped the known elements into categories he called metals, nonmetals, gases, and earths. Mendeleev’s Periodic Table How did Mendeleev organize the elements in his periodic table? Periodic Table Lab: Have a go at it! • Answer the pre-lab questions – come show Mr. Hill to get the cards • Use the card to do as Mendeleev did… • Get the rest of the cards • Answer the post-questions with your group • HW – Answer the extension questions – Mendeleev: SciShow Mendeleev’s Periodic Table Periods Mendeleev arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column. Groups Mendeleev’s Periodic Table Mendeleev’s Proposal In the 1860s, Dmitri Mendeleev developed an approach for organizing the elements while playing the card game solitaire. Mendeleev’s Periodic Table Mendeleev made a “deck of cards” of the elements, listing an element’s name, mass, and properties on each card. When Mendeleev lined up the cards in order of increasing mass, a pattern emerged. The key was to break the elements into rows. Mendeleev’s Periodic Table The final arrangement was similar to a winning arrangement in solitaire, except that the columns were organized by properties instead of suits. Within a column, the masses increased from top to bottom. Mendeleev’s chart was a periodic table. A periodic table is an arrangement of elements in columns, based on a set of properties that repeat from row to row. Mendeleev’s Periodic Table Evidence Supporting Mendeleev’s Table What evidence helped verify the usefulness of Mendeleev’s table? Mendeleev’s Periodic Table Mendeleev’s Prediction Gaps in the table! Mendeleev was able to predict the properties of an element based on its location in his table. He used the properties of elements located near the blank spaces in his table to predict properties for undiscovered elements. Mendeleev’s Periodic Table The close match between Mendeleev’s predictions and the actual properties of new elements showed how useful his periodic table could be. Mendeleev’s Periodic Table – Example of Predictability Mendeleev named missing elements after elements in the same group. He gave the name “eka-aluminum” to the missing element one space below aluminum in the table. Mendeleev predicted that “eka-aluminum” would • be a soft metal, • have a low melting point, and • have a density of 5.9 g/cm3. Mendeleev’s Periodic Table - Example of Predictability In 1875, a French chemist discovered a new element. He named the element gallium (Ga) in honor of France. (The Latin name for France is Gallia.) Gallium • is a soft metal, • has a melting point of 29.7°C, and • has a density of 5.91 g/cm3. Mendeleev’s Periodic Table…Gallium randomness Heat from a person's hand can melt gallium. In some traffic signals, there are tiny light emitting diodes (LEDs) that contain a compound of gallium. Mendeleev’s Periodic Table Scientists use the periodic table to explain the chemical behavior of different groups of elements. • The discovery of scandium (Sc) in 1879 • The discovery of germanium (Ge) in 1886 = More evidence. Assessment Questions 1. In Mendeleev’s periodic table, elements with similar properties were grouped a. b. c. d. in the same row. in the same column. in diagonal lines that run from top left to the bottom right. in pairs of two. Assessment Questions 1. In Mendeleev’s periodic table, elements with similar properties were grouped a. b. c. d. in the same row. in the same column. in diagonal lines that run from top left to the bottom right. in pairs of two. ANS: B Assessment Questions 2. For which element did Mendeleev correctly predict the properties even before it had been discovered? a. b. c. d. gallium hydrogen bromine aluminum Assessment Questions 2. For which element did Mendeleev correctly predict the properties even before it had been discovered? a. b. c. d. gallium hydrogen bromine aluminum ANS: A 5.2 Take the Tour! Take the Tour! The eight-note interval between any two notes on a keyboard with the same name is an octave. The sounds of musical notes that are separated by an octave are related, but they are not identical. In a similar way, elements in the same column of the modern periodic table are related but not identical. The Periodic Law CrashCourse How is the modern periodic table organized? The Periodic Law How is the modern periodic table organized? In the modern periodic table, elements are arranged by increasing atomic number (number of protons). The Periodic Law How is the modern periodic table organized? In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. The Periodic Law The modern periodic table is based on atomic number, or number of protons. The Periodic Law Periods Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy level. • This pattern applies to all the elements in the first column on the table. The Periodic Law Groups Each column in the periodic table is called a group. • The elements in a group have similar electron configurations, so members of a group in the periodic table have similar chemical properties. • This pattern of repeating properties is the periodic law. The Periodic Law Periodic Table of the Elements Atomic Mass What does the atomic mass of an element depend on? Atomic mass is a value that depends on the distribution of an element’s isotopes in nature and the masses of those isotopes. Atomic Mass Atomic Mass Units The mass of an atom in grams is extremely small. In order to have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard. • Scientists assigned 12 atomic mass units to the carbon-12 atom, which has 6 protons and 6 neutrons. • An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom. Atomic Mass There are four pieces of information for each element. Atomic Mass There are four pieces of information for each element. Atomic number Atomic Mass There are four pieces of information for each element. Atomic number Element symbol Atomic Mass There are four pieces of information for each element. Atomic number Element symbol Element name Atomic Mass There are four pieces of information for each element. Atomic number Element symbol Element name Atomic mass Atomic Mass Isotopes of Chlorine In nature, most elements exist as a mixture of two or more isotopes. The element chlorine has an atomic mass of 35.453 amu. Where does the number 35.453 come from? • There are two natural isotopes of chlorine, chlorine35 and chlorine-37. • An atom of chlorine-35 has 17 protons and 18 neutrons. • An atom of chlorine-37 has 17 protons and 20 neutrons. Atomic Mass Weighted Averages This table shows the atomic masses for the two naturally occurring chlorine isotopes. The value of the atomic mass for chlorine is a weighted average. If you add the atomic masses of the isotopes and divide by 2, you get 35.967, not 35.453. Classes of Elements What categories are used to classify elements on the periodic table? Classes of Elements Elements are classified as metals, nonmetals, and metalloids. Classifying Elements Three different ways to classify elements in the P-Table 1. State: solid—black symbol, liquid—purple symbol, or gas—red symbol 2. Occurrence in nature: elements that do not occur naturally— symbol. 3. General properties: metal—blue background, nonmetal— yellow background, or metalloid—green background Metals Most common type Metals are elements that are good conductors of electric current and heat. • Except for mercury, metals are solids at room temperature. • Most metals are malleable. • Many metals are ductile; that is, they can be drawn into thin wires. Classes of Elements The metals in groups 3 through 12 are called transition metals. Transition metals are elements that form a bridge between the elements on the left and right sides of the table. • Transition elements, such as copper and silver, were among the first elements discovered. • One property of many transition metals is their ability to form compounds with distinctive colors. RANDOM SIDE NOTE ERBIUM + Oxygen A compound used to tint the pink glass lenses. Elton John’s Dog Classes of Elements Nonmetals Nonmetals generally have properties opposite to those of metals. • Nonmetals are elements that are poor conductors of heat and electric current. • Nonmetals have low boiling points–many nonmetals are gases at room temperature. • Nonmetals that are solids at room temperature tend to be brittle. If they are hit with a hammer, they shatter or crumble. Classes of Elements Fluorine is the most reactive nonmetal. The gases in Group 18 are the least reactive elements in the table. Some toothpastes use a compound of the nonmetal fluorine and the metal sodium to help prevent tooth decay. Classes of Elements Metalloids Metalloid elements are located on the periodic table between metals and nonmetals. • Metalloids are elements with properties that fall between those of metals and nonmetals. • For example, a metalloid’s ability to conduct electric current varies with temperature. Silicon (Si) and germanium (Ge) are good insulators at low temperatures and good conductors at high temperatures. Variations Across a Period How do properties vary across a period in the periodic table? Variations Across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in their properties. Variations Across a Period From left to right across Period 3, there are three metals (Na, Mg, and Al), one metalloid (Si), and four nonmetals (P, S, Cl, and Ar). Variations Across a Period • Sodium reacts violently with water. • Magnesium will not react with water unless the water is hot. • Aluminum does not react with water, but it does react with oxygen. • Silicon is generally unreactive. • Phosphorus and sulfur do not react with water, but they do react with oxygen. • Chlorine is highly reactive. • Argon hardly reacts at all. Assessment Questions 1. What determines the atomic mass of an element? a. the natural distribution of isotopes and the atomic numbers of those isotopes b. the natural distribution of isotopes and the masses of those isotopes c. the mass of the isotope of the element that has the most neutrons d. the average number of protons in the element’s nucleus Assessment Questions 1. What determines the atomic mass of an element? a. the natural distribution of isotopes and the atomic numbers of those isotopes b. the natural distribution of isotopes and the masses of those isotopes c. the mass of the isotope of the element that has the most neutrons d. the average number of protons in the element’s nucleus ANS: B Assessment Questions 2. Which of the following is not characteristic of metals? a. b. c. d. ductile good electrical conductor typically solid at room temperature brittle Assessment Questions 2. Which of the following is not characteristic of metals? a. b. c. d. ductile good electrical conductor typically solid at room temperature brittle ANS: D Assessment Questions 3. Within a period of the periodic table, how do the properties of the elements vary? a. b. c. d. Metallic characteristics increase from left to right. Metallic characteristics decrease from left to right. Reactivity increases from left to right. Reactivity decreases from left to right. Assessment Questions 3. Within a period of the periodic table, how do the properties of the elements vary? a. b. c. d. Metallic characteristics increase from left to right. Metallic characteristics decrease from left to right. Reactivity increases from left to right. Reactivity decreases from left to right. ANS: B Assessment Questions 1. In the modern periodic table, elements are arranged in order of increasing atomic mass. True False Assessment Questions 1. In the modern periodic table, elements are arranged in order of increasing atomic mass. True False ANS: F, atomic number