Unit 2: Section A - River Dell Regional School District
advertisement

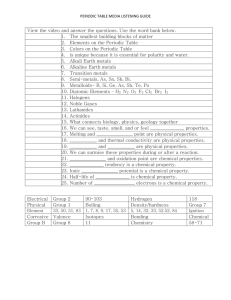
Unit 2: Section A: Why We Use What We Do Objectives: Distinguish between chemical and physical properties and between chemical and physical changes Classify specific examples as either chemical or physical properties Classify specific examples as either chemical or physical changes Classify selected elements as metals, nonmetals or metalloids based on observation of chemical and physical properties Use the Periodic Tale to: a) predict physical and chemical properties of an element (based on location) b) identify elements by their atomic masses and atomic number c) locate periods and groups (families) of elements Distinguish between isotopes based on their total neutrons and/or mass numbers. Calculate the mass number for an atom based on its total protons and neutrons. Explain how an element’s chemical and physical properties are associated with the number and arrangement of electrons in its atoms. Vocabulary/Terms Introduced: Define each term physical properties chemical properties physical change chemical change combustion luster ductile metals nonmetals metalloids conductor nonconductor malleable or brittle atomic number nucleus mass number isotopes average atomic mass periods periodic relationship group or family alkali metal family alkaline earth metals halogen family transition metals noble gas family Demonstrations: Copper in nitric acid (chemical and physical properties) Laboratory Activities: A.5 Investigating Matter: Metal or NonMetal? Reference Sheets: Periodic Table (See attached on School Wires) In Class: Check school wires and complete the worksheet “Differences of Metals and Nonmetals.” Worksheets: Isotopes, ions and atoms. ChemCom —Terms –Questions Assigned Read A.1-A.2 pg. 94-97—Define: physical properties, chemical properties, physical change, chemical change, combustion, luster, ductile. Questions #1-9 pg. 95-96, Section B Summary Questions #1-7 pg. 109. Read A.3-A.4 pg. 97-101—Define: metals, nonmetals, metalloids, conductor, nonconductor, malleable, brittle. Do Section A Summary Questions #8-11 pg. 109. In Class: Check school wires and complete the worksheet “Differences of Metals and Nonmetals.” Read A.6 (skip A.7) A.6 pg. 101-107. Do Section A Summary Questions #12-16 pg. 110 on the Periodic Table. Read A.8 pg. 107. Answer questions # 1-6. Define: Atomic number, nucleus, mass number, isotopes, and average atomic mass. Finish defining terms