Notes - Castle High School
advertisement

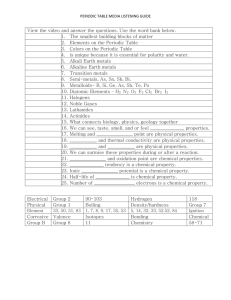
5.2 The Modern Periodic Table The eight-note interval between any two notes on a keyboard with the same name is an octave. The sounds of musical notes that are separated by an octave are related, but they are not identical. In a similar way, elements in the same column of the modern periodic table are related but not identical. How is the modern periodic table organized? In the modern periodic table, ______________________________________________________________________________ __________________________________________________________________________________________________________________ Properties of elements repeat in a ____________________________________________ when atomic numbers are used to arrange elements into groups. The modern periodic table is based on atomic number, or number of protons. Periods Each row in the table of elements is a __________________________________. •Hydrogen, the first element in _______________________, has one electron in its ___________________________________ level. •Lithium, the first element in _________________________, has one electron in its ___________________________________ level. •Sodium, the first element in __________________________, has one electron in its ___________________________________ level. •This pattern applies to all the elements in the first column on the table. Groups Each column in the periodic table is called a _______________________________________. •The elements in a group have similar electron configurations, so members of a group in the periodic table have _________________________________________________________________________________________. •This pattern of repeating properties is the ___________________________________________________________. 5.2 The Modern Periodic Table What does the atomic mass of an element depend on? Atomic mass is a value that depends on the _____________________________________________________________ __________________________________________________________________________________________________________________ Atomic Mass Units The mass of an atom in grams is extremely small. In order to have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard. •Scientists assigned 12 atomic mass units to the carbon-12 atom, which has 6 protons and 6 neutrons. •An atomic mass unit (amu) is defined as ______________________________ the mass of a carbon-12 atom. There are four pieces of information for each element. Isotopes of Chlorine In nature, most elements exist as ____________________________________________________________________________. The element chlorine has an atomic mass of 35.453 amu. Where does the number 35.453 come from? •There are two natural isotopes of chlorine, _______________________________________________________________. •An atom of chlorine-35 has ________________________________________________________________________________. •An atom of chlorine-37 has _________________________________________________________________________________ 5.2 The Modern Periodic Table Weighted Averages This table shows the atomic masses for the two naturally occurring chlorine isotopes. The value of the atomic mass for chlorine is a _______________________________________. If you add the atomic masses of the isotopes and divide by 2, you get _______________________________________________________________________. What categories are used to classify elements on the periodic table? Elements are classified as __________________________________________________________________________________ The periodic table presents three different ways to classify elements. •State: solid—____________________ symbol, liquid—_____________________ symbol, or gas—_________symbol •Occurrence in nature: elements that do not occur naturally—_____________________ symbol. •General properties: metal—______________ background, nonmetal—__________________ background, or metalloid—_______________________ background Metals The majority of the elements on the periodic table are classified as ____________________________. Metals are elements that are ______________________________________________________ of electric current and heat. •Except for mercury, _________________________________________________________________________________________. •Most metals are _______________________________. •Many metals are _______________________________; that is, they can be drawn into thin wires. A When magnesium reacts with oxygen, a dull layer forms on its surface. The layer can be removed to reveal magnesium’s shiny surface. B Many telescope mirrors are coated with aluminum to produce a surface that reflects light extremely well. The metals in groups 3 through 12 are called transition metals. _________________________________________ are elements that form a bridge between the elements on the left and right sides of the table. •Transition elements, such as copper and silver, were among the ________________________________________ •One property of many transition metals is their ability to form compounds with __________________________________________________________. A compound of oxygen and the transition element erbium is used to tint the pink glass lenses. Nonmetals Nonmetals generally have properties ___________________________________ to those of metals. •_____________________________ are elements that are ____________________________________________ of heat and electric current. •Nonmetals have ____________________________________________–many nonmetals are _____________________ at room temperature. •Nonmetals that are solids at room temperature ______________________________________________________. If they are hit with a hammer, they shatter or crumble. Fluorine is the most reactive nonmetal. The gases in Group 18 are the _________________________________ elements in the table. Some toothpastes use a compound of the nonmetal fluorine and the metal sodium to help prevent tooth decay. 5.2 The Modern Periodic Table Metalloids Metalloid elements are located on the periodic table _____________________________________________________ _________________________________________________________________________________________________________________. •_____________________________________ are elements with properties that fall between those of metals and nonmetals. •For example, a metalloid’s ability to conduct electric current varies with temperature. Silicon (Si) and germanium (Ge) are good insulators at low temperatures and good conductors at high temperatures. How do properties vary across a period in the periodic table? Across a period _____________________________________________________________________________________________ _________________________________________________________________________________________________________________ From left to right across Period 3, there are three metals (Na, Mg, and Al), one metalloid (Si), and four nonmetals (P, S, Cl, and Ar). •Sodium _____________________________________________________ with water. •Magnesium will not react with water unless the water is hot. •Aluminum does not react with water, but it does react with oxygen. •Silicon is generally unreactive. •Phosphorus and sulfur do not react with water, but they do react with oxygen. •Chlorine is highly reactive. •Argon ______________________________________________________________________ at all. 1. What determines the atomic mass of an element? a.the natural distribution of isotopes and the atomic numbers of those isotopes b.the natural distribution of isotopes and the masses of those isotopes c.the mass of the isotope of the element that has the most neutrons d.the average number of protons in the element’s nucleus 2. Which of the following is not characteristic of metals? a.ductile b.good electrical conductor c.typically solid at room temperature d.brittle 3. Within a period of the periodic table, how do the properties of the elements vary? a.Metallic characteristics increase from left to right. b.Metallic characteristics decrease from left to right. c.Reactivity increases from left to right. d.Reactivity decreases from left to right. 1. In the modern periodic table, elements are arranged in order of increasing atomic mass. True False