day 3 focus 6.P.2
advertisement

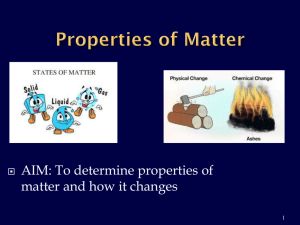
Sit in assigned seats No talking Studying for your quiz quietly Water can exist on Earth in 3 different states of matter. Explain these 3 different states of matter and detail how the atoms move in each state. Remember to brainstorm first, then construct your sentences. One unique property of water is that it can exist in 3 different states on planet Earth. Water can exist as a solid in the ice caps of the North Pole, as a liquid in rivers and stream, and as a gas in the atmosphere. In a solid, atoms are packed tightly together. In a liquid, atoms are free to move around only slightly. In a gas, atoms move around rapidly to fill in the space. Physical change Solubility Condensation Temperature Melting Point Chemical Change Evaporation Density Solute / Solvent Boiling Point DO NOT WRITE ON THIS QUIZ ….THIS IS A CLASS SET! YOU WILL BE USING YOUR PAPER- TAKE OUT A SHEET OF PAPER NOW AND WRITE YOUR NAME THERE ARE 15 MULTIPLE CHOICE QUESTIONS Take out a sheet of paper NUMBER FROM 1 – 15 Eliminate answer choices, make your best educated guess if you are unsure…. Check over all your answers and make sure you answer every question! YOU WILL 1:30 FOR EACH QUESTION WRITE ALL INFORMATION IN YOUR ALPHABET OF SCIENCE BOOKLET Properties of matter DO NOT depend on the amount of matter that makes up an object. Properties in 8 small in pieces of iron iron = Properties 1 large piece of Shape is a characteristic property of matter: an object will retain all of its characteristic properties regardless of its shape. both clear and colorless, have a boiling point at 100ºC, can dissolve table salt, etc. Matter has characteristic properties that can be divided into physical properties chemical properties “Property Changes” CAN BE MEASURED AND OBSERVED no chemical change to the substance Includes: color, odor, density, solubility, melting point, boiling point Example : wood cut in half both pieces still have the same physical properties Includes states of matter changes (solid, liquid, gas) due to changes in energy / temperature Watch the videos – you do not have to take notes “Solids to liquids and back again” New substances are formed! Includes properties such as acidity, basicity, combustibility, and reactivity. “Tablets reacting in solution” Nail rusting outside “Chemical and Physical Properties of Matter” Evaporation point: The point where liquid turns into a gas Condensation Point: The temperature at which a gas becomes a liquid Melting point: The temperature at which a solid becomes a liquid Boiling point: The temperature at which a liquid boils Density: A ratio that compares the mass of an object to its volume (dividing mass / volume) Object Sinks: when a object's density is greater than 1.0 gram / mL (density of water) Object Floats: when a object's density is less than 1.0 gram/ mL (density of water) Solubility: the ability of a substance to dissolve in another substance Solute: A substance that is dissolved in a solution Solvent: the substance that does the dissolving Water is known as the universal solvent because so many substances will dissolve in water! Tuesday: We will be using technology to review all the vocabulary for Standard 6.P.2, Also we will complete our marshmallow stations and everyone will create their own crossword puzzle Log in to Chromebooks…. First name initial last name last 4 digits of student id # @cms.gaggle.net Example: tmaas1234@cms.gaggle.net Password: year of birth, month of birth, date of birth Example: 010228 (2001, February 28) You will be going to the following website to complete several activities The address is: http://quizlet.com/