Solutions
Name: __________________________________________________ Per: __________
Learning Targets:
Describe the characteristics of a solution
Explain why water is called the universal solvent
Differentiate between saturated and unsaturated solutions
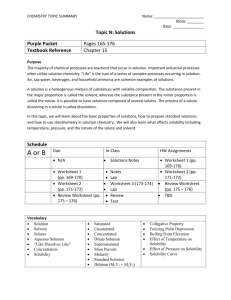
Date
1/5
1/6 & 1/7
1/8 & 1/9
1/12
1/13 & 1/14
1/15 & 1/16
1/20 & 1/21
1/22 & 1/23
1/26
1/27-1/29
Describe 4 ways to speed up the rate of dissolving
Identify the parts of a solution
Describe how the presence of a solute affects the freezing and boiling points of solution
Activities
Mini-Lab
Read/Lecture 5-1: What is a Solution?
Reading Questions
Read/Lecture 5-2: Parts of a Solution
Reading Questions
LAB: Solubility
Read/Lecture: 5-4: Rate Changes
Reading Questions
LAB: Rate of Solubility
QUIZ: Solutions, solution parts and rate changes
Read/Lecture: 5-3: Why is water a good solvent
Reading Questions
Read/Lecture: 5-5: The concentration of a solution
Reading Questions
LAB: Making Supersaturated Solutions
Read/Lecture: 5-6: How solutes affect freezing point
Read/Lecture: 5-7: How solutes affect boiling point
LAB: Boiling point of water versus salt water
Reading Questions
QUIZ: Water, concentrations, freezing and boiling points
Read/Lecture: 5-8: How can solutions be separated?
LAB: Evaporating solutions
Video: Distillation of petroleum
Reading Questions
Chapter Review
20 Questions
SEMESTER FINAL REVIEW
Study for finals
FINAL EXAM: ½ the test will be about solutions. The other ½ of the test will be questions from previous tests over: science skills, properties of matter, states of matter, atoms & elements and acids & bases
Vocabulary:
Solution
Solute
Solvent
Soluble
Solubility
Insoluble
Saturated solution
Unsaturated solution
Freezing point
Boiling point
Evaporation
Condensation
5-1: What is a solution?
Dissolve:
Why does salt seem to disappear in water?
Solution:
Why is air called a solution?
5-2: What are the parts of a solution?
Parts of a solution:
Soluble:
Solubility:
What will happen when a substance that is soluble in water is mixed with water?
Insoluble:
How can a substance be both soluble and insoluble?
5-4: How can you change the rate at which a substance dissolves?
Why does stirring make a sugar cube dissolve faster in water?
What is the relationship between the temperature of a liquid solvent and the rate at which the solid dissolves in it?
Which would dissolve faster in the same amount of water at the same temperature: a sugar cube or powdered sugar? Why?
Why do you think more gas can dissolve when pressure is increased?
What type of compounds will water dissolve?
5-3: Why is water a good solvent?
Why is a water molecule called a “polar molecule”?
What happens to the sugar molecule when sugar is placed in water?
What will happen if the force of attractions holding solute particles together is greater than the force of attraction between solute and solvent?
How are molecular solutions similar to ionic solution? How are they different?
5-5: What is the concentration of a solution?
Dilute Solution:
Concentrated solution:
Unsaturated solution:
Saturated solution:
If you were preparing a solution, how could you tell when it became saturated?
Supersaturated:
What happens to a solution that enables it to hold more solute than it normally would?
5-6: How do solutes affect freezing point?
Freezing point:
What happens to liquid water at its freezing point?
Freezing point depression:
What affects do you think the use of salts would have on plants and animals in the environment?
5-7: How do solutes affect boiling points?
Boiling point:
What happens when the temperature of pure water reaches 100 degrees Celsius?
Boiling point elevation:
What two important functions does an engine coolant have?
5-8: How can solutions be separated?
Evaporation:
Explain how to separate salt from salt water:
Condensation:
Distillation:
What two processes are involved in distillation?