Formulas and Names for Covalent Compounds
advertisement
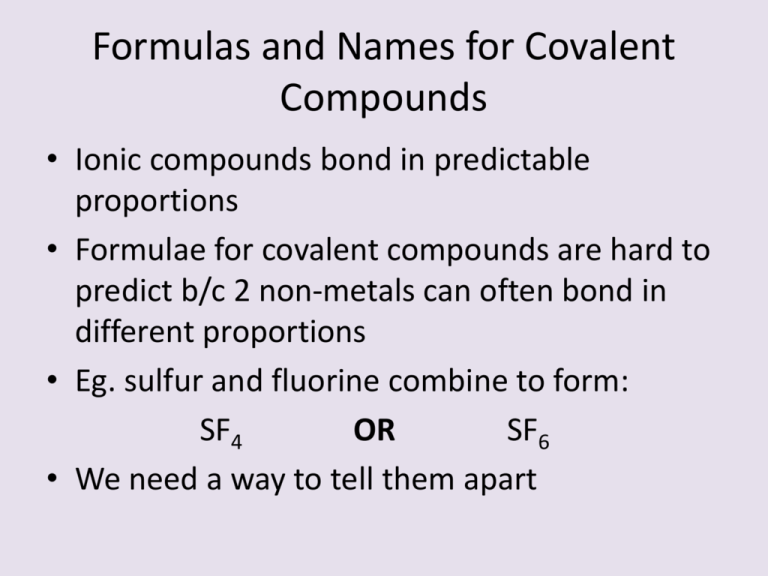
Formulas and Names for Covalent Compounds • Ionic compounds bond in predictable proportions • Formulae for covalent compounds are hard to predict b/c 2 non-metals can often bond in different proportions • Eg. sulfur and fluorine combine to form: SF4 OR SF6 • We need a way to tell them apart Naming Covalent Compounds • We add Greek prefixes to indicate the number of each element Prefix Number Prefix Number mono- 1 hexa- 6 di- 2 hepta- 7 tri- 3 octa- 8 tetra- 4 nona- 9 penta- 5 deca- 10 Naming Covalent Compounds • Write the less electronegative element first with the correct prefix • Write the second element, with the correct prefix • Change the ending of the second element to “ide”, just like ionic compounds • “mono-” is only used for the second element. If the first element has one atom, just use the element name Examples • • • • • • • • CO CO2 SCl2 SF4 SF6 N2O5 P2O3 P4O10 Formulas for Covalent Compounds • Use the prefixes to find the number of each atom present • Eg. disilicon hexabromide • Exceptions for covalent compounds H2O = water NH3 = ammonia CH4 = methane Formulas and Names for Binary Acids • Binary Acids – contain H and one other element • Many are gases at room temperature – Only when dissolved in H2O do they become acids • Use the prefix “Hydro” + second element name + suffix “ic”, followed by acid • Eg. HCl(aq) = hydrochloric acid Note: aq = aqueous Read as “in water” Examples of Binary Acids • HF(aq) • H2S(aq) • HCN(aq) Formulas and Names for Ternary Acids • • • • • • Ternary Acids contain oxyanions Oxyanions – polyatomic anions with oxygen Anions ending in “ate” change to “ic” Anions ending in “ite” change to “ous” Write “acid” after the anion name When writing chemical formulas (aq) must be written after the formula Examples • • • • • • HNO3 H2SO4 HClO4 HClO3 HClO2 HClO = = = = = = nitric acid sulfuric acid perchloric acid chloric acid chlorous acid hypochlorous acid Try These • HNO2 • H3PO4 • H2CO3