IBS Chemistry Objectives Unit 1: Chem 1 Review
advertisement

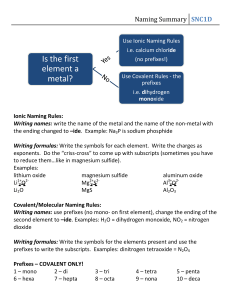
IBS Chemistry Objectives Unit 1: Chem 1 Review Chapters: 1,2 Objectives 1. Using the periodic table a. Identify families (alkali metals, noble gases, etc) b. Give number of electrons, protons, neutrons of element c. Describe basic structure of atom (electron cloud, energy levels, nucleus, etc) 2. Nomenclature and formulas (SOL Ch. 3 a, c) a. Distinguish between empirical, molecular, and structural formulas b. Name covalent compounds using the Greek prefix system of mono, di, tri etc. c. Write chemical formulas given the name of a compound d. Choose the appropriate naming rules for a given chemical formula e. Learn the names and formulae of common anions and cations f. Write chemical formulas for ionic compounds given i. Name of compound or ii. A pair of ions g. Identify polyatomic ions h. Classify compounds as being ionic or covalent. i. Name ionic compounds using stock system (Roman numerals) 3. Lab Safety and Techniques (SOL Ch 1. a, b, c) a. Identify, locate, and know how to use laboratory safety equipment including aprons, goggles, gloves, fire extinguishers, fire blanket, safety shower, eye wash, broken glass container, and fume hood. b. Decide which safety and emergency procedures to follow in case of particular accidents including fires and hazardous material spills. c. Demonstrate the following basic lab techniques: using analytical balance (tare), obtaining chemicals without contamination, filtering, decanting, using chromatography, lighting gas burners. d. Demonstrate proper methods for carrying and moving chemicals and equipment. 4. Data Measurement and Manipulation a. Use dimension analysis to convert quantities from one metric unit to another, and also between metric and English units. b. Propagate errors in simple calculations c. Read a measurement from a graduated scale, stating measured digits plus the estimated digit. d. Report the degree of uncertainty of a measurement e. Determine the significant digits in a recorded measurement, and carry out mathematical operations using these measurements with answers rounded off to the correct number of significant digits. f. Report very large and very small numbers in scientific notation g. Perform mathematical operations on numbers in scientific notation h. Use data collected to calculate percent error. 5. Experiment Design a. Design and perform experiments to test predictions b. Design specific procedures to control variables c. Start to become familiar with IB lab methods