Writing Formulas for Covalent Compounds Name Period ___ Date ___
advertisement
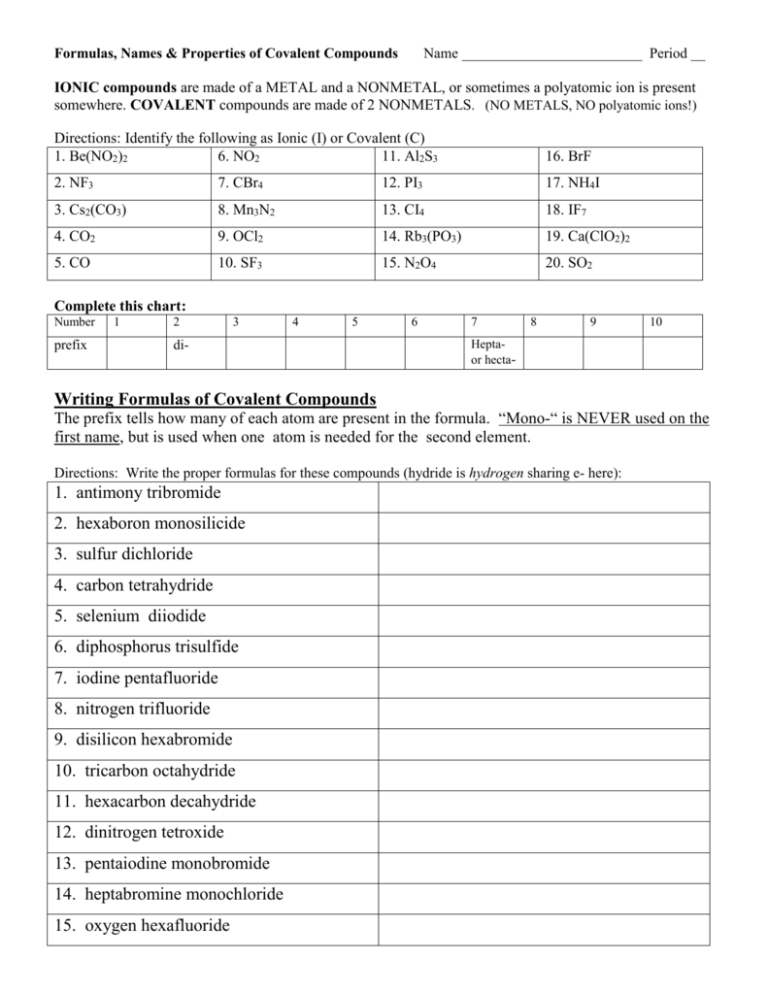
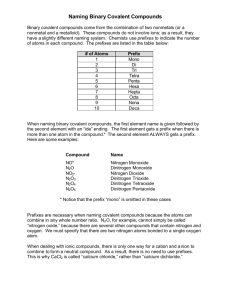
Formulas, Names & Properties of Covalent Compounds Name _________________________ Period __ IONIC compounds are made of a METAL and a NONMETAL, or sometimes a polyatomic ion is present somewhere. COVALENT compounds are made of 2 NONMETALS. (NO METALS, NO polyatomic ions!) Directions: Identify the following as Ionic (I) or Covalent (C) 1. Be(NO2)2 6. NO2 11. Al2S3 16. BrF 2. NF3 7. CBr4 12. PI3 17. NH4I 3. Cs2(CO3) 8. Mn3N2 13. CI4 18. IF7 4. CO2 9. OCl2 14. Rb3(PO3) 19. Ca(ClO2)2 5. CO 10. SF3 15. N2O4 20. SO2 Complete this chart: Number prefix 1 2 3 4 5 di- 6 7 8 9 10 Heptaor hecta- Writing Formulas of Covalent Compounds The prefix tells how many of each atom are present in the formula. “Mono-“ is NEVER used on the first name, but is used when one atom is needed for the second element. Directions: Write the proper formulas for these compounds (hydride is hydrogen sharing e- here): 1. antimony tribromide 2. hexaboron monosilicide 3. sulfur dichloride 4. carbon tetrahydride 5. selenium diiodide 6. diphosphorus trisulfide 7. iodine pentafluoride 8. nitrogen trifluoride 9. disilicon hexabromide 10. tricarbon octahydride 11. hexacarbon decahydride 12. dinitrogen tetroxide 13. pentaiodine monobromide 14. heptabromine monochloride 15. oxygen hexafluoride Naming Covalent Compounds / prefix naming system Use the prefixes ONLY on covalent compounds. See the examples! FIRST NAME = write the prefix (except mono- is not used on the 1st name ) and the name of the element SECOND NAME = always gets a prefix (mono IS used when 1 atom is there) and change the ending to “-ide”. Ex 1. SN2 Sulfur dinitride Ex 3. C7I4 Heptacarbon tetraiodide Ex 2. CSi carbon monosilicide Ex 4. I2O 17. NO 27. I2O7 18. N2O5 28. I2O5 19. N2O 29. AtCl5 20. NO2 30. I5Cl 21. P2O5 31. I7Cl 22. P2O3 32. SbCl5 23. Cl2O7 33. AsBr3 24. Cl2O5 34. Ge3N4 25. SeS 35. P3N5 26. SeS2 36. TeF6 diiodine monoxide Use your notes here! Classify the following properties as belonging to Ionic compounds or Covalent compounds 1. HIGH melting point 4. Low boiling point 7. Has a crystalline 10. is an electrolyte (crystal ) shape 2. conducts electricity 5. conducts electricity 8. Low melting point 11. is a nonelectrolyte when dissolved when melted 3. does not dissolve well 6. does not conduct 9. dissolves well 12. High boiling point electricity