Naming Covalent Compounds
advertisement
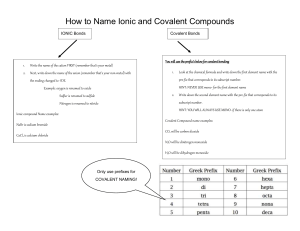
Naming Covalent Compounds Objectives: 1. The name for covalent compounds with given formula 2. Give a formula for covalent compounds with given name Covalent Cmpds • Made of 2 anions (2 negatively charged ions) • Identify the less EN element of the two (left and down— decreases) • The 1st nonmetal is given the name on the PT • The second changes the ending to –ide…don’t change polyatomic • Never use “criss-cross” to balance • Use prefixes to show how many of each element are need • Number • Prefix • 1 • Mono • 2 • Di • 3 • Tri • 4 • Tetra • 5 • Penta • 6 • Hexa • 7 • Hepta • 8 • Octa • 9 • Nona • 10 • Deca Using prefixes • Never use if the first element only has one Practice SO2 sulfur dioxide N2O dinitrogen monoxide NO2 nitrogen dioxide CCl4 carbon tetrachloride Cl2O7 dichlorine heptaoxide PCl3 phosphorus trichloride SF6 sulfur hexafluoride Si3N4 trisilicon tetranitride N(BrO3)5 nitrogen pentabromate H2O water • • • • • • • • Phosphorus trioxide Dinitrogen pentacarbide Tellurium noniodide Carbon monoxide Selenium heptafluoride Tetraphosphorous decoxide Arsenic hexabromide Silicon dichloride PO3 N2C5 TeI9 CO SeF7 P4O10 AsBr6 SiCl2