Light and Electron Test Review
advertisement

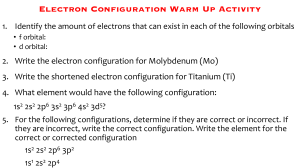
Light and the Electron Test Review Waves 1. Label the letters on the wave pictured to the right. 2. Order the waves below from lowest frequency to highest frequency (Hint-some may be equal). Wave and Light Equations What is the speed of light equal to? What is Planck’s Constant equal to? 1. What is the wavelength of light that has a frequency of 4.22 x 1015 Hz? 2. What is the energy of light that has a frequency of 1.30 x 1014 Hz? 3. What is the frequency of a wave of light with the wavelength of 450 nm. 4. 0.825 M = _______ nm 5. The wavelength of a certain beam of light was 3.52x10-7m. a) Find the frequency of this light. b) Calculate how much energy this light has. Light Emissions The diagram to the right shows 4 possible electron emissions. Use the diagram to answer the following questions. 1. Is energy being absorbed or released in the diagram? How do you know? 2. There are four wavelengths present in the diagram: violet, blue, green, and red. Which atom represents each color? 3. Explain the difference between an atom in its ground state and an atom in its excited state. Orbital Diagrams Draw an orbital diagram for the following atoms in their ground state. 1. 2. 3. 4. 5. Chlorine Chromium Neon Helium Phosphorus Draw an orbital diagram for the following electron configurations. Then identify which element each electron configuration represents. 6. 7. 8. 9. 10. 1s2 2s2 2p6 3s2 3p6 1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 3s1 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 1s2 2s2 2p6 3s2 3p6 4s2 3d7 Draw an orbital diagram that represents the following elements in an excited state. 11. Chlorine 12. Chromium 13. Neon Electron Configurations Write the extended electron configurations for the following elements. 1. 2. 3. 4. 5. Fluorine Copper Lithium Silicon Vanadium Write the abbreviated electron configurations for the following elements. 6. 7. 8. 9. 10. Osmium Cesium Silver Bromine Polonium Electromagnetic Spectrum Draw an electromagnetic spectrum, including a zoomed-in section for visible light. Label each category of light in the correct order. Be sure to label all the colors of the visible light in order. Vocabulary Create your own vocabulary list for the words you think are important to know in this unit. Each word should be accompanied by a definition or explanation. You need to have at least 10 words in your list. Name________________________ ____ Date_____________ Light and Electron Review Answer Sheet Waves 1. 2. Wave and Light Equations C= h= 1. 2. 3. 4. 5. a. b. Light Emissions 1. 2. 3. Orbital Diagrams 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Electron Configurations 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Electromagnetic Spectrum Vocabulary List 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20.