Chemistry I: Electron Configurations Worksheet
advertisement
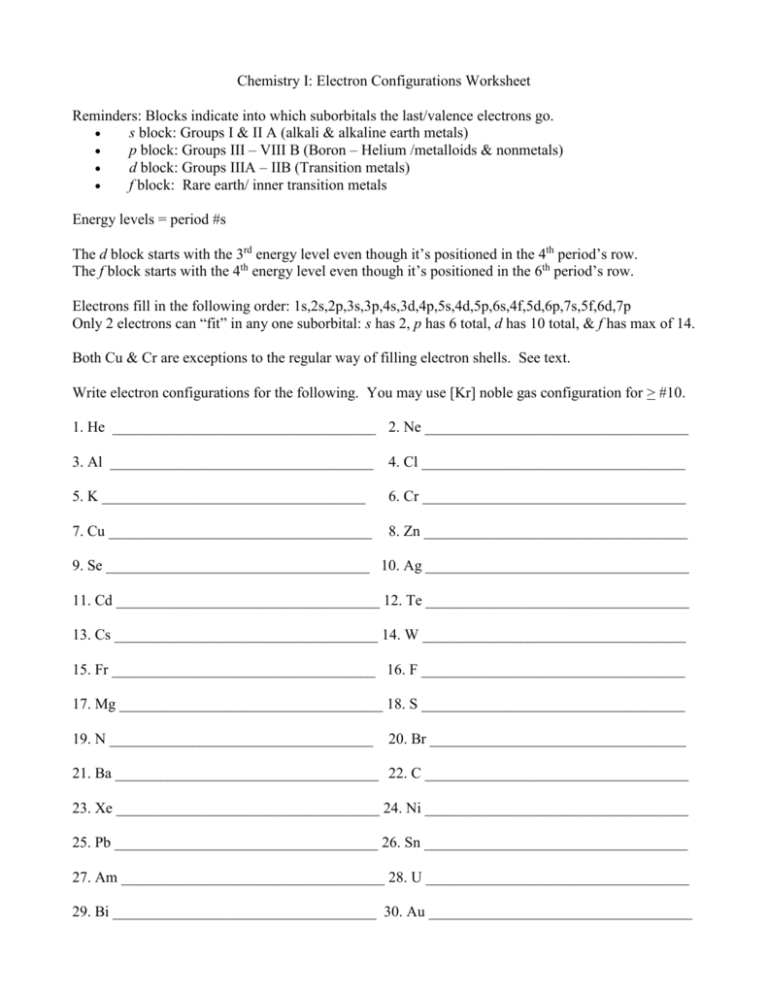
Chemistry I: Electron Configurations Worksheet Reminders: Blocks indicate into which suborbitals the last/valence electrons go. s block: Groups I & II A (alkali & alkaline earth metals) p block: Groups III – VIII B (Boron – Helium /metalloids & nonmetals) d block: Groups IIIA – IIB (Transition metals) f block: Rare earth/ inner transition metals Energy levels = period #s The d block starts with the 3rd energy level even though it’s positioned in the 4th period’s row. The f block starts with the 4th energy level even though it’s positioned in the 6th period’s row. Electrons fill in the following order: 1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p Only 2 electrons can “fit” in any one suborbital: s has 2, p has 6 total, d has 10 total, & f has max of 14. Both Cu & Cr are exceptions to the regular way of filling electron shells. See text. Write electron configurations for the following. You may use [Kr] noble gas configuration for > #10. 1. He ___________________________________ 2. Ne ___________________________________ 3. Al ___________________________________ 4. Cl ___________________________________ 5. K ___________________________________ 6. Cr ___________________________________ 7. Cu ___________________________________ 8. Zn ___________________________________ 9. Se ___________________________________ 10. Ag ___________________________________ 11. Cd ___________________________________ 12. Te ___________________________________ 13. Cs ___________________________________ 14. W ___________________________________ 15. Fr ___________________________________ 16. F ___________________________________ 17. Mg ___________________________________ 18. S ___________________________________ 19. N ___________________________________ 20. Br __________________________________ 21. Ba ___________________________________ 22. C ___________________________________ 23. Xe ___________________________________ 24. Ni ___________________________________ 25. Pb ___________________________________ 26. Sn ___________________________________ 27. Am ___________________________________ 28. U ___________________________________ 29. Bi ___________________________________ 30. Au ___________________________________