Ch. 20 - Electrochemistry Review WS
advertisement
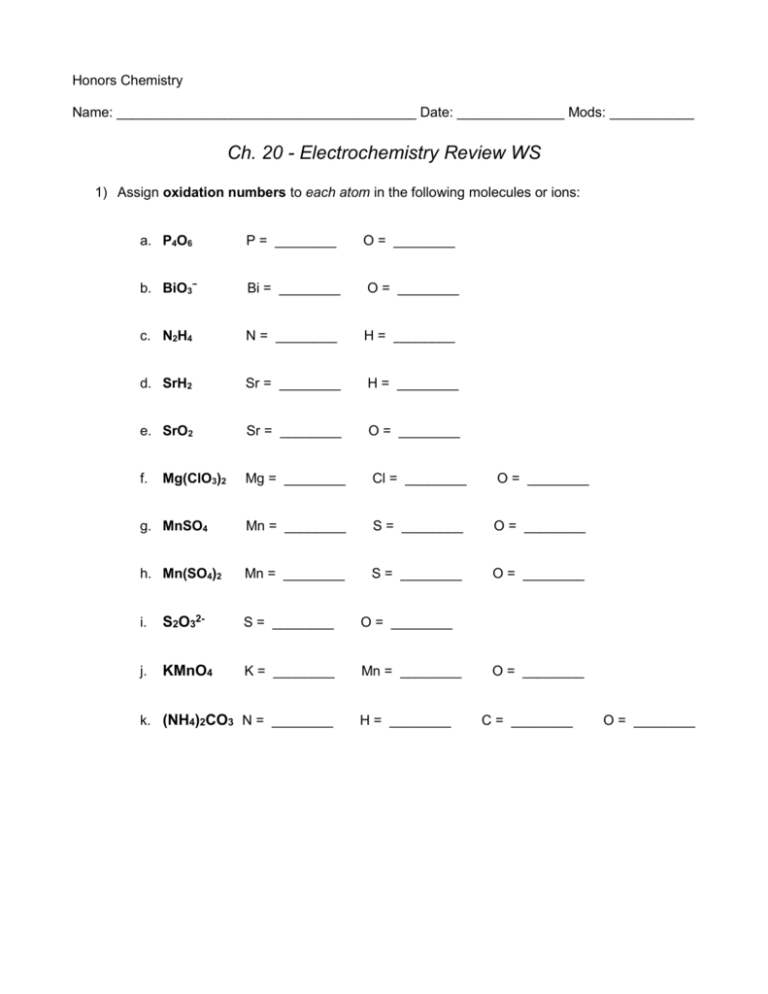
Honors Chemistry Name: _______________________________________ Date: ______________ Mods: ___________ Ch. 20 - Electrochemistry Review WS 1) Assign oxidation numbers to each atom in the following molecules or ions: a. P4O6 P = ________ O = ________ b. BiO3− Bi = ________ O = ________ c. N2H4 N = ________ H = ________ d. SrH2 Sr = ________ H = ________ e. SrO2 Sr = ________ O = ________ f. Mg = ________ Cl = ________ O = ________ g. MnSO4 Mn = ________ S = ________ O = ________ h. Mn(SO4)2 Mn = ________ S = ________ O = ________ Mg(ClO3)2 i. S2O32- S = ________ O = ________ j. KMnO4 K = ________ Mn = ________ k. (NH4)2CO3 N = ________ H = ________ O = ________ C = ________ O = ________ 2) For each of the reactions below identify what is being oxidized, what is being reduced, the oxidizing agent and the reducing agent. Finally, write the net ionic reactions. a. SiCl4 (aq) + 2 Mg (s) 2 MgCl2 (aq) + Si (s) _______ is oxidized ; ___________ is the oxidizing agent ; _______ is reduced ___________ is the reducing agent Net Ionic: b. 2 Al (s) + 3 F2(g) 2 AlF3 (s) _______ is oxidized ___________ is the oxidizing agent ; ; _______ is reduced ___________ is the reducing agent Net Ionic: 3) Determine if the following half reactions are oxidation or reduction balance each half by adding the appropriate number of electrons. a. Ti (s) Ti4+ (aq) b. S4O6 (g) S2O32- (aq) 4) Balance each of the following oxidation-reduction reactions by the half-reaction method. These reactions each occur in an ACIDIC solution. a. Fe2+ (aq) + MnO4- (aq) Fe3+ (aq) + Mn2+ (aq) b. H2O2 (l) + Cr2O72- (aq) O2 (g) + Cr3+ (aq) 5. Given the cell notation below and using the activity series, determine which electrode will act as the cathode and which will act as the anode and then write the balanced oxidation-half and reduction-half reactions. Al (s) | Al 3+ (aq) ǁ Sn2+ (aq) | Sn (s) a. Cathode = _______ ; Anode = _______ b. Which electrode will have a decrease in mass after the redox reaction takes place?______ c. Balanced Oxidation-half reaction: d. Balance Reduction-half reaction: 6. Draw a voltaic cell which would be developed from the two half-reactions below. Label the anode and the cathode, show the direction of electron flow, include a K+/Cl- salt bridge, and beakers which include the aqueous solutions present in each half-cell (assume both cells use nitrate (NO3–) solutions). Half-Reactions: Pb2+ (aq) + 2e– Pb (s) and Cr (s) Cr3+ (aq) + 3e–