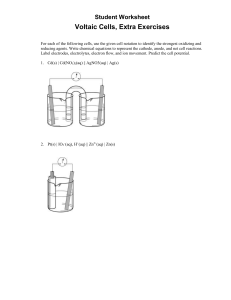
Name______________________________ Period___________ Assignment For each of the following: Label the cathode, anode, electron movement, ion movement, and write the half reactions taking place at each half cell. Describe one observation that could be made at each half cell that would indicate the cell is functioning. #1 #2 Draw a diagram of the following cells. Include the labels and equations as above. #3 Ag|Ag+ ||Fe2+|Fe3+ #4 Zn/Zn+2 Mg/Mg+2 For each of the following cells, use the given cell notation to identify the strongest oxidizing and reducing agents. Write chemical equations to represent the cathode, anode, and net cell reactions. Label electrodes, electrolytes, electron flow, and ion movement. 1. Cd(s) | Cd(NO3)2(aq) || AgNO3(aq) | Ag(s)