Galvanic Cells Worksheet: AP Chemistry Practice
advertisement

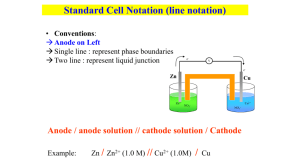
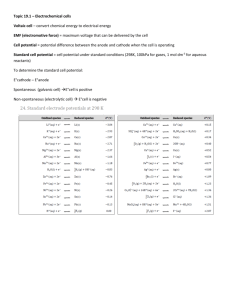
AP Chemistry Chapter 17 Section 1, 2 & 4 Galvanic Cells 1. Using the half-reactions given answer the questions below: Half – Reaction E (V) Ce+4(aq) + e- Ce+3(aq) Ag+(aq) + e- Ag(s) Hg2+2(aq) + 2e- 2Hg(l) Sn+2(aq) + 2e- Sn(s) Ni+2(aq) + 2e- Ni(s) Al+3(aq) + 3e- Al(s) +1.61 + .80 + .79 - .14 - .25 - 1.66 a. Which is the weakest oxidizing agent in the table? ___________ b. Which is the strongest reducing agent? __________ c. Will Sn reduce Ag+ to Ag? _________ d. Will Hg reduce Sn+2 to Sn? _________ 2. Using the table in #1, answer the following: a. Write a balanced equation for the reaction leading to the maximum positive potential. b. If the Ni/Ni+2 half cell is combined with the Hg/Hg+2 half cell, write the spontaneous reaction that would occur. Calculate the E of this cell. 3. Assume that you assemble an electrochemical cell based on the half reactions: Ce 3+(aq), Ce4+(aq) and Sn(s), Sn2+(aq). Answer the following questions: a. Write the balanced equation for the reaction that occurs spontaneously. b. Which species acted as the oxidizing agent? ___________________ c. Write the line notation for the cell. ______________________ d. Diagram the cell. Label all parts. 4. The unit of measurement for the potential of a galvanic cell is the ________. 5. True or False: The measured voltage of a working electrochemical cell is always positive. 6. In the standard half cell the E on the notation E represents _____________ 7. In the standard half cell the on the notation E represents _____________ 8. What two species are present in the standard half cell for oxidation or reduction to take place. 9. Write the two reactions possible in the standard half cell and state which would occur when the half cell is acting as the anode and which would occur when the half cell is acting as the cathode. a. __ b. __ 10. Which three of the following are standard conditions for the half cell: 0.0 C, 25 C, 100.0 C, 0.10 M, 1.0M, 10.0 M, 0.01 atm, 0.10 atm, 1.0 atm 11. In the Fe/Fe2+ // Cu2+/Cu voltaic cell which electrode is acting as the anode? Which electrode would be gaining in mass? 12. Using the standard reduction potential table, which would be the better oxidizing agent, H2O2 or Zn2+? 13. What is the significance of a standard reduction potential that has a negative value? 14. Calculate the Ecell and indicate if the SHE half cell will gain e- (cathode) or lose e- (anode) when the SHE half cell is connected to half cells containing the following at standard conditions. a. Ag in Ag+ b. Cl- in Cl2 c. Cu in Cu2+ d. Zn in Zn2+ e. Mg in Mg2+ f. Fe in Fe3+