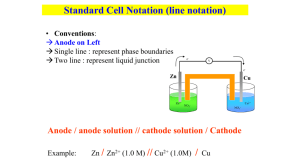
Electrochemistry Worksheet: Cell Potential & Redox Reactions
advertisement
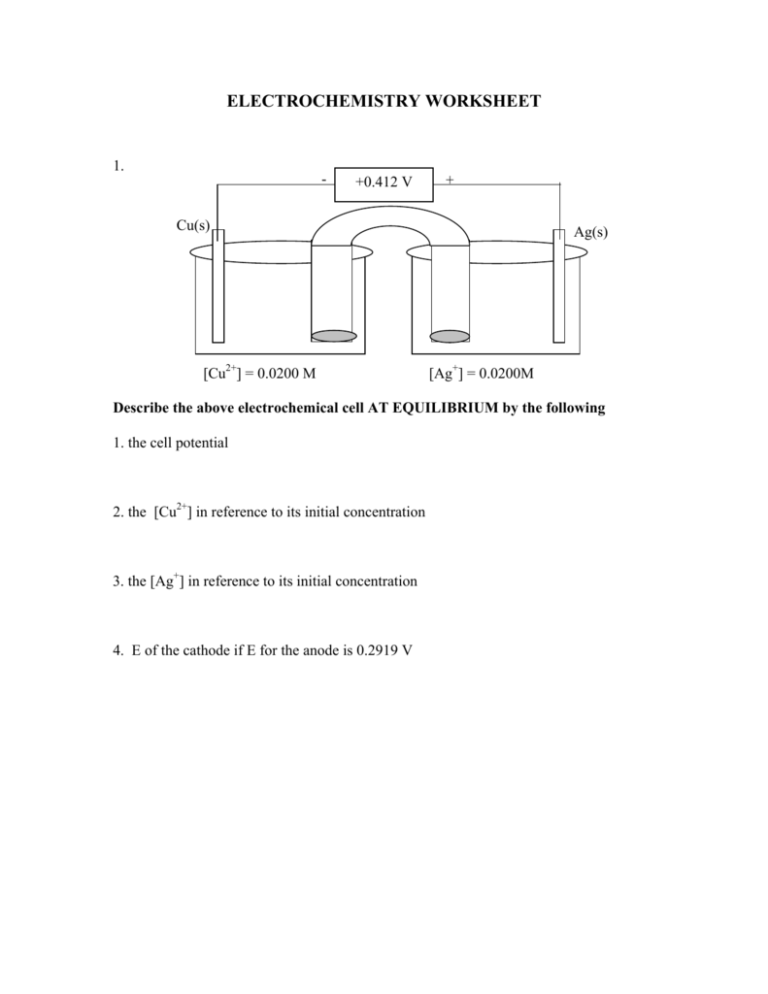
ELECTROCHEMISTRY WORKSHEET 1. - +0.412 V + Cu(s) Ag(s) AgNO3 (aq) [Cu2+] = 0.0200 M [Ag+] = 0.0200M Describe the above electrochemical cell AT EQUILIBRIUM by the following 1. the cell potential 2. the [Cu2+] in reference to its initial concentration 3. the [Ag+] in reference to its initial concentration 4. E of the cathode if E for the anode is 0.2919 V 2. Calculate the potential of a Pt electrode immersed in a solution prepared by mixing 25.00 mL of 0.0918 M SnCl2 with 25.00 mL of 0.1568 M FeCl3. 3. - +0.546 V + KCl Pt(s) Ag(s) HCl(aq) 1.0 M 0.0151 M FeCl2 (aq) ?????? M FeCl3 (aq) AgCl(s) 1. Write the balanced half reaction that is occurring at the anode 2. Write the balanced half reaction that is occurring at the cathode 3. Write the overall cell reaction 4. What is the concentration of Fe3+ in this cell. 4. Bi(OH)3(s) + SnO22-(aq) Bi(s) + SnO32- ; BASE solution a.Write the half reaction for the cathode? b.Write the half reaction for the anode? c. Write the balanced redox reaction. d. What species is the reducing agent in this oxidation reduction reaction? POSSIBLE USEFUL INFORMATION E0 for Cl2(g)/ Cl- = +1.359 V E0 for Cr+3/ Cr(s) = -0.744 V E0 for Cr+3/ Cr+2 = -0.408 V E0 for Fe2+/ Fe(s) = -0.440 V E0 for Fe3+/ Fe2+ = +0.771 V E0 for AgBr(s)/Ag = +0.073 V E0 for Ag+/ Ag(s) = +0.799 V E0 for SO42-/ H2SO3 = +.172 V E0 for Sn2+/ Sn(s) = -0.136 V E0 for Sn4+/ Sn2+ = +0.154 V E0 for Zn+2/Zn(s) = -0.763 ∆G = -nFE ∆G = -Eq F = 9.649 x 104 C/mol eE = E0 - (0.0592/n) log ([C]c[D]d)/([A]a[B]b) Ksp Zn(OH)2 = 3.0 x 10-6