14.2b Cell Potentials
advertisement

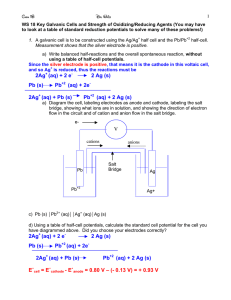
14.2b Cell Potentials Reduction Potential Cell Potential cell -- standard cell potential max. electrical potential difference of a standard cell difference between the cathode and anode energy driving force on electron to move them through the wire also called electromotive force (emf) units are Volts (V) o 1 V = 1 J/coulombs of charge An Electrochemical Process Involves Electron Transfer at the Interface Between the Electrode and the Solution Digital Voltmeters Cell potential con’t or -- standard reduction potential ability of a standard half-cell to attract electrons (reduction) the half-cell with the more positive red. potential gains eo E cell o =E o r cathode - E r anode Standard Reduction Potentials we assign r values to each half-reactions o we can find the total cell by taking the difference between the reduction potentials for the two halfreactions Rules do not change the sign of r if you flip the half-rxtn o do not multiply the r by an integer used to balance the electrons o If ocell is positive the net rxtn is spontaneous (therefor voltaic/galvanic) Standard Reduction Potentials ° values for reduction half-reactions with all solutions having 1 M conc and all gases 1 atm Standard Hydrogen Electrode used as reference half-cell to determine all other o r values Pt electrode in contact with 1 M H+ and H2(g) at 1 atm assigned an r of zero can calculate others by pairing with this and measuring total cell o 2H+(aq) + 2e-(aq) H2(g) r o = 0.00 V Standard Hydrogen Electrode Example Using a cadium-zinc cell and given cell notation, label electrodes & determine cell potential. Cd(s) | Cd2+(aq) || Zn2+(aq) | Zn(s) a) Cd(s) | Cd2+(aq) || Zn2+(aq) | Zn(s) cathode o b) E anode o cell =E r cathode - o Er anode (from data booklet, red. potential table) = (-0.40V) - (-0.76V) o E cell = + 0.36 V (spont/voltaic) Homework Textbook p631 #10,11 P633 #12,13,14 LSM 14.2 F & G