Compare the properties of esters with carboxylic acids
advertisement

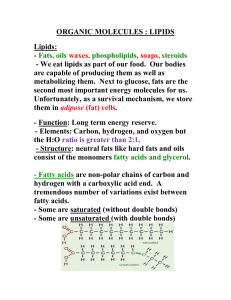
ESTERS What is an ester? • Responsible for tastes and odours. • Contain the ester linkage O C R R' O • Made from an alcohol and a carboxylic acid. • Compare the properties of esters with carboxylic acids NAMING • Use the “yl” form of the alcohol proceeded with the “oate” form of the carboxylic acid. i.e. “alkyl oate” • Compare the name of the ester to that of the alcohol and acid from which it was synthesize. What is the pattern? Examples: a) ethyl butanoate ==> CH3CH2COOCH2CH3 b) 3-methylbutyl ethanoate ==> CH3COOCH2CH2CH(CH3)CH3 Reactions of Esters 1. Preparation H S O / 2 4 c a r b o x y l i c a c i d + a l c o h o l HO H HO H O H O CH+ HCC 2 H S O / 2 4 H C HCC + H O H O H H e t h a n o i c a c i d ( a c e t i c a c i d ) 2. e s t e r+ w a t e r H H m e t h y l e t h a n o a t e ( m e t h y l a c e t a t e ) m e t h a n o l Hydrolysis (reverse of 1) e s t e r+ e x c e s s w a t e r H S O / 2 4 a l c o h o l+ c a r b o x y l i c a c i d H OH O H H H S O / 2 4 H C H+ C H O H+ O C C C H C H 2 H O H O H H H m e t h y l e t h a n o a t e ( m e t h y l a c e t a t e ) m e t h a n o l e t h a n o i c a c i d ( a c e t i c a c i d ) • For the above reactions, if there is an excess of the alcohol and carboxylic acid, reaction 1 is favoured and if there is an excess of water, reaction 2 will be favoured. 3. Irreversible Hydrolysis Basic Solution ester + water H H O H C CO C H H H methylethanoate (methylacetate) • + H2O NaOH alcohol + carboxylate ion O H H C C H H C H + -O H O H Na+ methanol sodium ethanoate (sodium acetate) Since these conditions produce a stable carboxylate ion, the reaction is not reversible Waxes • Waxes are mixtures of esters having carbon chains in the range of 16 to 34 carbons on each side of the ester linkage. Example O C H3C(H2C)7 (CH2)29CH3 O Beeswax • Since the hydrocarbon chains are so long, waxes are very water repellant. • Biological Importance: fruit, leaves, insects Fats and Oils • Fats and oils are triesters of glycerol that contain at least one fatty acid. • Fatty acids are carboxylic acids that contain 14-18 carbon and having varying degrees of unsaturation OH OH OH H C C C H H H H glycerol 1,2,3-propanetriol O CH3(CH2)15 C OH stearic acid glycerol + 1 fatty acid monoglyceride + water glycerol + 2 fatty acids diglyceride + water glycerol + 3 fatty acids triglyceride + water Fats in Plants • The hydrocarbon chains are unsaturated with 1,2, or 3 C=C bonds. • The double bonds reduce the intermolecular forces by causing “kinks” in the chain resulting in lower melting and boiling points. • For this reason the fats are generally liquid (oils) • Since the double bonds provide a site for reactions, the break down of unsaturated fats is more effective (i.e. less cholesterol). • The double bonds are easily oxidized meaning edible oils will go rancid (i.e. spoil) and oil based paints/varnish will crosslink to produce tough films. Fats in Animals • The hydrocarbon chains are fully saturated. • The chains are fairly straight allowing for close packing and greater intermolecular forces. • Due to the lack of unsaturation, they are not broken down easily by the body and can cause the build up of cholesterol. • Solids at room temperature. Salicylates • Contain salicylic acid. • Most common is acetyl salicyclic acid “ASA” (AKA aspirin) which is used for pain relief and as an antiseptic. HO O HO O HO O OH O salicylic acid acetyl salicylic acid ASA Saponification Fats and oils are esters of long chain acids, which can be heated with a strong base (NaOH) to produce acid salts which we call soap. This process is called saponification.