Let's Build Lipids (Ms. Gardner)
advertisement

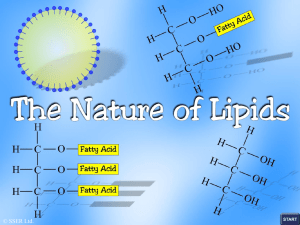
Name: ____________________________ Block: ___________ Let’s Build Lipids! Introduction Lipids are a diverse group of chemical compounds that are related by their insolubility in water. Lipids include phospholipids, sterols, and triglycerides. Phospholipids are important parts of cell membranes. Sterols such as cholesterol form vital biological compounds including hormones. Triglycerides store energy, protect certain organs, transport fatsoluble vitamins, and help insulate the body. Triglycerides are the most common type of lipid found in the body and in foods. Triglycerides include the edible fats and oils in our diets - substances such as olive oil, corn oil, peanut oil, butterfat, and lard. Triglycerides that are solid or semisolid at room temperature are classified as fats, and occur predominantly in animals. Those triglycerides that are liquid are called oils and originate chiefly in plants. Fats and oils are made up of two different kinds of molecules, glycerol and fatty acids. In this activity, you will (a) examine the molecular structure of glycerol and fatty acids. (b) use structural formulas and molecular models of glycerol and fatty acids to determine how these molecules join together to form fats and oils. The Structure of Glycerol The molecular formula for Glycerol is: CH2OHCHOHCH2OH. What type of molecule is glycerol? How do you know? Draw the structural formula of Glycerol: Now, build it! Using the Play-Doh and toothpicks, build a model of glycerol using the structural formula you constructed above. Building Up and Breaking Down Fatty acids are the second kind of molecule found in triglycerides. Saturated fatty acids have no double bonds between the carbon atoms of the fatty acid chain and all other bonds are attached to hydrogen atoms. An unsaturated fatty acid does have one or more double bonds between carbon atoms. Now, build it! Carry out DEHYDRATION SYNTHESIS for the following saturated and unsaturated fats with Play-Doh. Build the individual molecules first and then put the molecules together via dehydration synthesis: 1. Glycerol + 3(CH3CH2CH2COOH) What are your products? ___________________ + ___________ How many water molecules are formed when one triglyceride molecule is produced? _____________ 2. Glycerol + 2 CH3CH2CH2COOH + 1 CH3CH2CHCHCH2COOH What are your products? ___________________ + ___________ How many water molecules are formed when one triglyceride molecule is produced? _____________ Carry out HYDROLYSIS for each of the previous structures you built above with Play-Doh. Put together the entire triglyceride first AND THEN break it apart. 1. What are your products? ___________________ + ___________ How many water molecules were used to form the triglyceride product? _____________ 2. What are your products? ___________________ + ___________ How many water molecules were used to form the triglyceride product? _____________