Solutions and Pure Solvents: How Can You Tell the Difference
advertisement
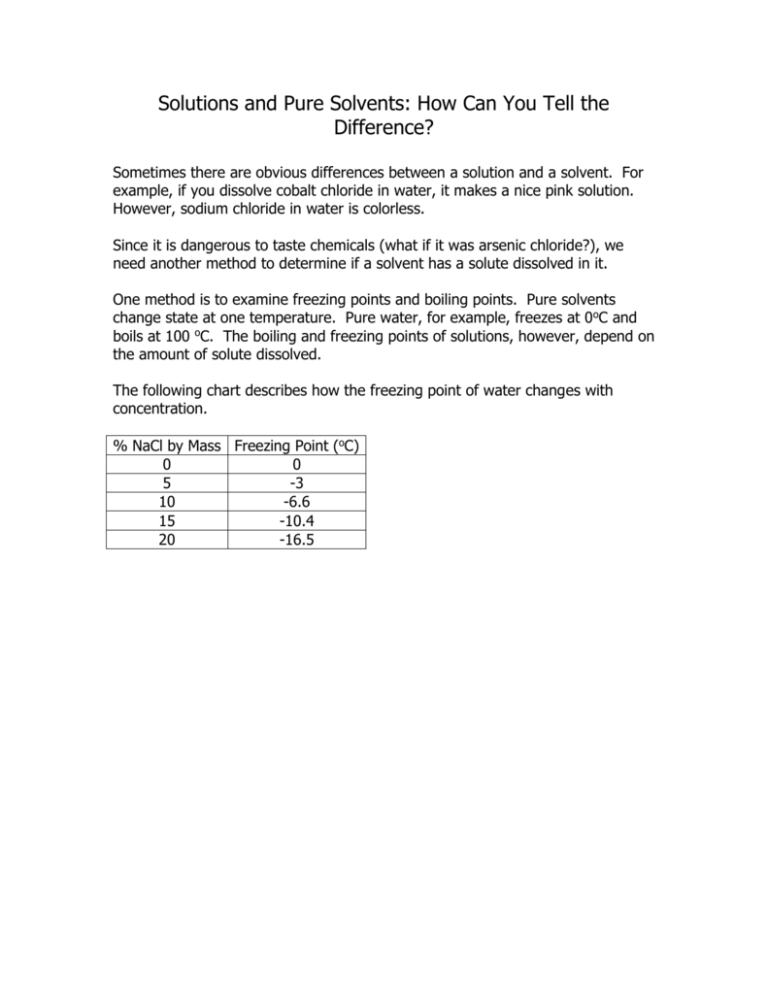
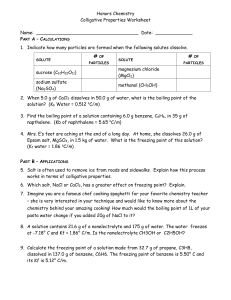
Solutions and Pure Solvents: How Can You Tell the Difference? Sometimes there are obvious differences between a solution and a solvent. For example, if you dissolve cobalt chloride in water, it makes a nice pink solution. However, sodium chloride in water is colorless. Since it is dangerous to taste chemicals (what if it was arsenic chloride?), we need another method to determine if a solvent has a solute dissolved in it. One method is to examine freezing points and boiling points. Pure solvents change state at one temperature. Pure water, for example, freezes at 0oC and boils at 100 oC. The boiling and freezing points of solutions, however, depend on the amount of solute dissolved. The following chart describes how the freezing point of water changes with concentration. % NaCl by Mass Freezing Point (oC) 0 0 5 -3 10 -6.6 15 -10.4 20 -16.5