Freezing Point Depression & Boiling Point Elevation
advertisement

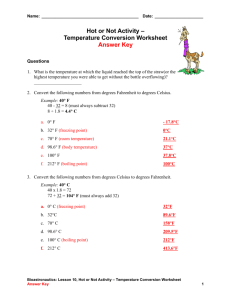
Freezing Point Depression & Boiling Point Elevation When a solution contains a non-volatile solute, the freezing point of the solution will be lower than the freezing point of the pure solvent. Similarly, with a nonvolatile solute, the boiling point of the solution will be higher than the boiling point of the solvent. These effects are called freezing point depression and boiling point elevation, respectively. These effects are colligative properties - properties that depend on the number of particles dissolved in a solution rather than the type of particle. Two other colligative properties are vapor pressure lowering and osmotic pressure. Freezing Point Depression Δ Tf = i X m X Kf Where: Δ Tf is the change in temperature of the freezing point in degrees Celsius. i is the van't Hoff factor (see below). m is the molality of the solutions (mols total solutes/kg solvent) Kf is the freezing point depression constant for the solvent. Boiling Point Elevation Δ Tb = m X Kb Where: Δ Tb is the change in temperature of the boiling point in degrees Celsius. i is the van't Hoff factor (see below). m is the molality of the solutions (mols solute/kg solvent) Kb is the boiling point depression constant for the solvent. van't Hoff Factor (i) Adjusts the equations for the total number of moles of solute. For molecules that do not dissociate into ions in solution (organics), i = 1. For ions, this equals the number of ions dissociated per molecule. Ex. For NaCl, i = 2. For MgCl2, i = 3. For Al2(SO4)3, i = 5. Example What mass of ethylene glycol (EG -> C2H6O2), in grams, must be added to 1.0 kg of water to produce a solution that boils at 105.0 C? Solution 1) We look up the Kb of water => 0.512 C/m. 2) We calculate the molecular weight of ethylene glycol as 62.07 g/mol. 3) i will equal 1 since ethylene glycol will not dissociate into ions. 4) The normal boiling point of water is 100 C. 5) Use the boiling point elevation equation, rearranged to calculate m. m = Δ Tb / iKb = (105 - 100) / (1)(0.512) = 9.77 5) 1.0 kg water x 9.77 mol EG x 62.07 g EG = 1 kg water 1 mol EG 610 g EG